There is limited understanding of shrimp immune responses to viral pathogens
Viral diseases have become one of the largest impediments to expansion of the commercial shrimp-farming industry worldwide. For example, the white spot syndrome virus (WSSV) has caused billions of dollars in losses since becoming endemic in most shrimp-farming regions. In addition, new viral diseases are continuing to emerge and cause tremendous impacts.
Current strategies for viral disease mitigation in farms have been focused on pathogen exclusion through specific pathogen-free stocks, development of disease-resistant animals and strict biosecurity programs. Commercially proven vaccines for shrimp are not yet available in the marketplace. This is mainly because a cost-effective delivery system has not been developed, and there is limited understanding of shrimp immune responses to viral pathogens.
Replicon particles
Alphavirus-derived replicon particles (R.P.) have been developed as a safe, flexible and rapid vector approach for animal vaccines. The authors have applied this technology to shrimp viruses to assess the feasibility of delivering a viral vaccine to shrimp.
The R.P. vector has numerous advantages for vaccine development, including accurate production of native proteins, a rapid turnaround process and a propagation-defective nature that stops any possibility of vaccine spreading from the immunized animals. In addition, alphaviruses are often transmitted to arthropods via oral infection, so oral vaccination of shrimp with R.P. is supported by this natural exposure route.
Developmental shrimp vaccine
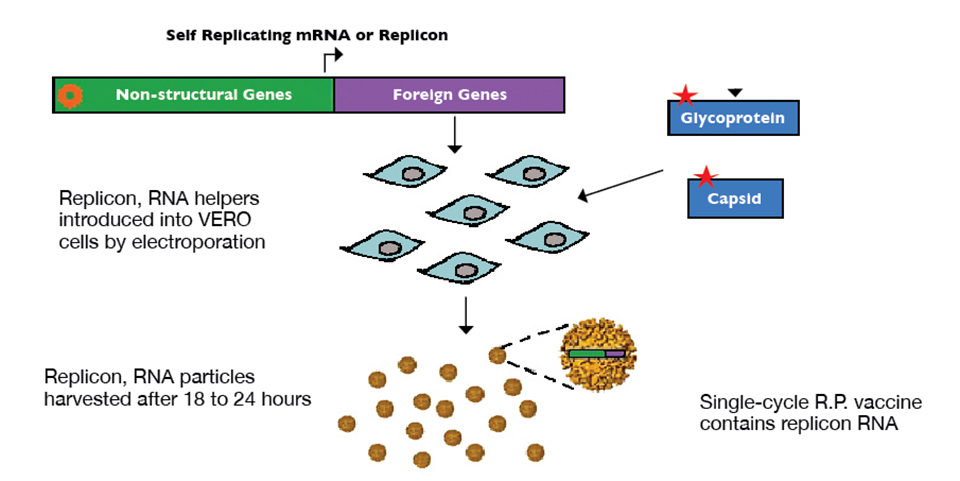
Overexpression of WSSV envelope proteins VP19 and VP28 using an alphavirus replicon particle was the basis for constructing a developmental shrimp vaccine for WSSV. Particles were synthesized by inserting sequences of the proteins into DNA plasmids, encoding a replication-incompetent alphavirus backbone replicon particle.
Replicon RNA containing VP19 or VP28 sequencing was transcribed in vitro and combined with helper RNA containing sequences for alphavirus structural genes in trans. These RNAs were then introduced into vero cells via electroporation, and replicon particles were harvested and purified following an 18- to 24-hour incubation period (Fig. 1).
WSSV challenge
To evaluate these vaccines, specific pathogen-free juvenile L. vannamei weighing 3 to 5 g were divided into treatment groups of 10 shrimp with three replicates per treatment. The experimental groups received VP19 R.P. or VP28 R.P. via injection into the third abdominal segment. The positive control group was injected with an equivalent volume of 2 percent sodium chloride.
After 72 hours, all the treatment groups were challenged by injection with WSSV, and the negative control group was injected with an equivalent volume of 2 percent sodium chloride. Mortality was observed for 21 days with dead or moribund animals immediately removed from tanks.
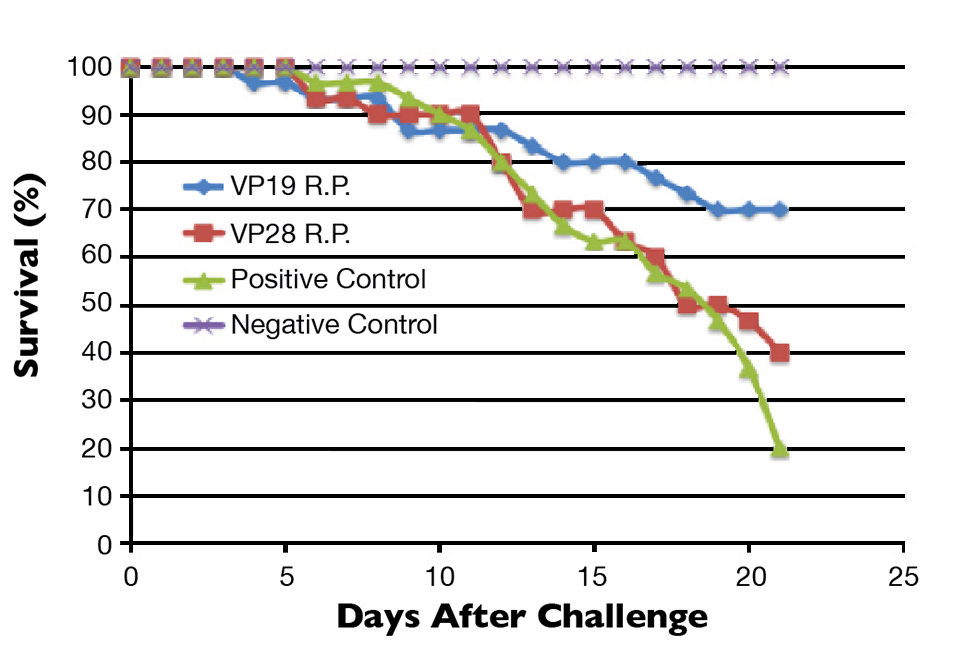
At termination of the experiment, VP19 R.P. and VP28 R.P. showed 70 percent and 40 percent survival, respectively. The positive control group had 20 percent survival (Fig. 2). WSSV infection was confirmed via immunohistochemistry and quantitative real-time polymerase chain reaction testing.
Perspectives
This study demonstrated that VP19 and VP28 expressed by R.P. provided protection against mortality due to WSSV. R.P. vaccination strategies hold promise for a field-deliverable product. In addition, the flexibility and rapid turnaround of the R.P. platform would enable a vaccine to quickly be developed for a variety of shrimp viral pathogens as they emerge or reemerge.
(Editor’s Note: This article was originally published in the May/June 2011 print edition of the Global Aquaculture Advocate.)
Authors
-
J. Dustin Loy, DVM
Research Scientist
Harrisvaccines, Inc.
1102 Southern Hills Drive, Suite 101
Ames, Iowa 50010 USA[109,111,99,46,115,101,110,105,99,99,97,118,115,105,114,114,97,104,64,121,111,108,100]
-
Duan S. Loy, DVM
Graduate Research Assistant
Iowa State University
Ames, Iowa, USA
Tagged With
Related Posts

Health & Welfare
CENIACUA develops WSSV-resistant shrimp in Colombia
To combat white spot syndrome virus (WSSV) in white shrimp, Corporación Centro de Investigación de la Acuacultura de Colombia (CENIACUA) initiated a selective-breeding program to develop resistance in shrimp.
Health & Welfare
Double-stranded RNA against WSSV genes provides antiviral protection in shrimp
Silencing genes in white spot syndrome virus (WSSV) with critical roles in replication could provide a strong antiviral effect and thus reduce shrimp mortality. The authors therefore established a study to evaluate the antiviral efficacy of double-stranded (ds)RNA against non-structural WSSV genes.

Health & Welfare
Effective management of WSSV in shrimp
As research on WSSV continues, commonsense steps can lessen the potential impacts of white spot syndrome virus on shrimp-farming operations.

Innovation & Investment
Patent law affects innovation in aquaculture
A virus that causes pancreas disease was patented, but when a vaccine produced by a patent licensee was found insufficient, another company developed a vaccine based on a different strain of the virus. The courts in Norway determined the work on the second vaccine was a patent law infringement.


