Results suggest BFT water can be reused at least for a second year

In this study, when biofloc water from a just completed experiment to produce market-size catfish was re-used immediately to hold those fish over the winter, ammonia transformation in response to an ammonia‑nitrogen spike was inversely related to mean water temperature from 3 to 12 degrees-C. No lag in ammonia biotransformation was observed when feeding of fish resumed in mid-March at daily rations ranging from 47 to 69 grams per cubic meter and resultant changes in NH4-N and NO2-N concentrations were < 0.1 mg per liter.
Based on previous studies, re-using biofloc water for more than one production cycle or season obviates the time required to establish a new, fully functional biofloc with its associated NH4-N and NO2-N spikes. Few published studies were found on the re-use of biofloc water for a subsequent production cycle despite anecdotal reports, and no study was found on using one-year-old biofloc water for a second year of production.
The accumulation of minerals – particularly cadmium, copper and zinc – is a concern where culture water is re-used with little or no make-up water for multiple production cycles because mineral concentrations may reach toxic levels. In addition to mineral accumulation in intensive recirculating aquaculture system (RAS) water, minerals may bioaccumulate in culture animal tissue, particularly edible tissue. Although there is published data on pond-reared catfish fillet mineral concentration, data on mineral content of catfish grown in the BFT system is lacking.
This article – adapted and summarized from the original publication – presents the results of a study to compare the effect of new, unused water and year-old used biofloc water on catfish production characteristics and water quality in the BFT production system.
Study setup
A completely randomized design was used in triplicate, outdoor, wood-framed BFT tanks used for channel catfish production to evaluate the following treatments: 1) one-year old BFT water that contained low total suspended solids (TSS) concentration (Old-Lo treatment); 2) one-year old BFT water that contained high TSS concentration (Old-Hi treatment); and, 3) newly (New) established BFT water.

The used biofloc water originated from an initial study that evaluated solids control in catfish BFT culture, and this water then was re-used immediately to evaluate low- and high-TSS BFT water for holding market size fish through the winter, before finally being re-used immediately for the present study. The BFT system started at the beginning of this experiment utilized the new biofloc water. Old versus new biofloc waters, low- versus high-TSS old biofloc waters, and old versus new low-TSS waters were the planned comparisons evaluated.
Fingerling catfish (47.5 ± 0.8 g/fish; mean ± SD) were stocked into tanks at 13.5 fish per square meter (16 fish per cubic meter) and initial biomass averaged 0.76 ± 0.2 kg per cubic meter. Fish were fed a commercial formulated feed (32 percent crude protein, 2.5 percent lipid) to apparent satiation six days per week and the quantity recorded. Feed conversion ratio (FCR) was calculated for each tank as the total quantity of feed fed (dry matter basis) divided by the net (wet) weight of fish harvested.
For detailed information on the experimental design, tank management, fish, and feeding; fish, tissue, and feed sampling analyses; MIB and geosmin analyses; water quality analyses; and statistical analyses, refer to the original publication.
Results and discussion
Production variables did not differ significantly between New and Old (water) treatments, nor between the Old-Lo and Old-Hi treatments. Gross and net fish yield averaged 10.2 and 9.4 kg per cubic meter, respectively. Total feed consumption averaged 12.8 kg per cubic meter across treatments and no significant differences (P > 0.05) were detected among treatments for daily feed consumption throughout the experiment. Feed conversion ratio averaged 1.36. At harvest fish averaged 641.6 grams per fish and 69.0 percent of the fish population attained market size (454 grams per fish). Liver size (HSI), muscle ratio, intraperitoneal fat, and whole-body protein, lipid, and moisture did not differ significantly among treatments.
Biofloc production system water from a full season of catfish growout that was used to maintain market-size fish over the winter was used successfully in the present study for a second year of catfish food-fish production. This is the first report of using biofloc water from the previous year for a second year of production. Whether the mixotrophic BFT production system was started fresh (New treatment) or utilized water from the previous year that differed significantly in initial TSS and chlorophyll a concentrations (Old-Lo and Old-Hi treatments), catfish growth, feed performance and compositional indices all responded similarly.
Mean daily feed consumption was similar in all treatments throughout the present study despite differences in TSS concentrations among treatments and over time. Side-stream settling chambers were used to remove TSS from New and Old-Lo treatment tanks to maintain mean TSS from 300 to 400 mg per liter; mean TSS in the Old-Hi treatment increased to a maximum of 1,415 mg per liter. Catfish feed consumption and growth in a previous BFT production system did not differ significantly between the Control treatment where maximum TSS concentration ranged from 1,200 to 1,410 mg per liter and treatments where TSS concentrations were maintained at about 300 mg per liter. However, the 5 to 11 percent reduction in feed consumption and 7 percent reduction in fish size in the Control treatment suggest that elevated TSS concentration adversely affects catfish performance. Results from this study and a previous study suggest that determination of an optimal upper limit for TSS concentration still is needed.
Ammonia‑nitrogen and NO2-N concentrations remained consistently low throughout the experiment in the Old water treatments because nitrification was fully functional from the beginning, whereas the typical lag in onset of nitrification was observed in the New treatment. Un-ionized ammonia, estimated to comprise about 8 percent of the spike in NH4-N concentration in the New treatment during onset of nitrification at the prevailing water temperature and pH, would be less than the reported LC50 of 2.4 mg per liter of non-ionized ammonia for channel catfish.
The significantly higher mean NO3-N accumulation rates observed in the New and Old-Hi treatments compared to the Old-Lo treatments suggests nitrification in the latter treatment was impacted negatively. The overall significant difference in settling chamber water and solids discharge in Old-Lo treatment tanks compared to New treatment tanks does not explain fully the observed reduction in NO3-N accumulation rate. Examination on a monthly basis of NO3-N accumulation rate and settling chamber water and solids discharge provides additional insight.
Results from the present experiment are consistent with reported reductions in nitrification rate in response to TSS removal from shrimp BFT production system tanks. Additional research on the optimum proportion of old biofloc water needed to establish nitrification rapidly in a new production cycle would be beneficial.
Green, catfish BFT, Table 1
| Response (b) | Treatment: New | Treatment: Old-Lo | Treatment: Old-Hi | Treatment SEp | ANOVA Pr > F (c) |
|---|---|---|---|---|---|
| NH4-N | 0.21 | 0.15 | 0.16 | 0.03 | 0.359 |
| NO2-N | 0.39 (x) | 0.16 (xy) | 0.05 (y) | 0.07 | 0.046 |
| NO3-N | 69.46 (y) | 94.58 (y) | 133.66 (x) | 8.22 | 0.004 |
| PO4-P | 11.68 (z) | 20.20 (y) | 29.73 (x) | 1.70 | 0.001 |
| TSS | 355.49 (y) | 409.89 (y) | 986.67 (x) | 31.54 | <0.001 |
| Chl a | 929.5 | 1,432.2 | 1,649.2 | 167.3 | 0.055 |
| T Alk | 108.2 (x) | 94.6 (y) | 108.2 (x) | 2.9 | 0.025 |
(a) N = 3 replicate tanks.
(b) NH4-N, total ammonia-nitrogen, mg/L; NO2-N, nitrite-nitrogen, mg/L; NO3-N, nitrate-nitrogen, mg/L; PO4-P, soluble reactive phosphate, mg/L; total suspended solids, mg/L; Chl a, chlorophyll a, mg/m3; T Alk, total alkalinity, mg/L as CaCO3.
(c) ANOVA, Pr >F. LS means in the same row with different letters are different (P ≤ 0.05).
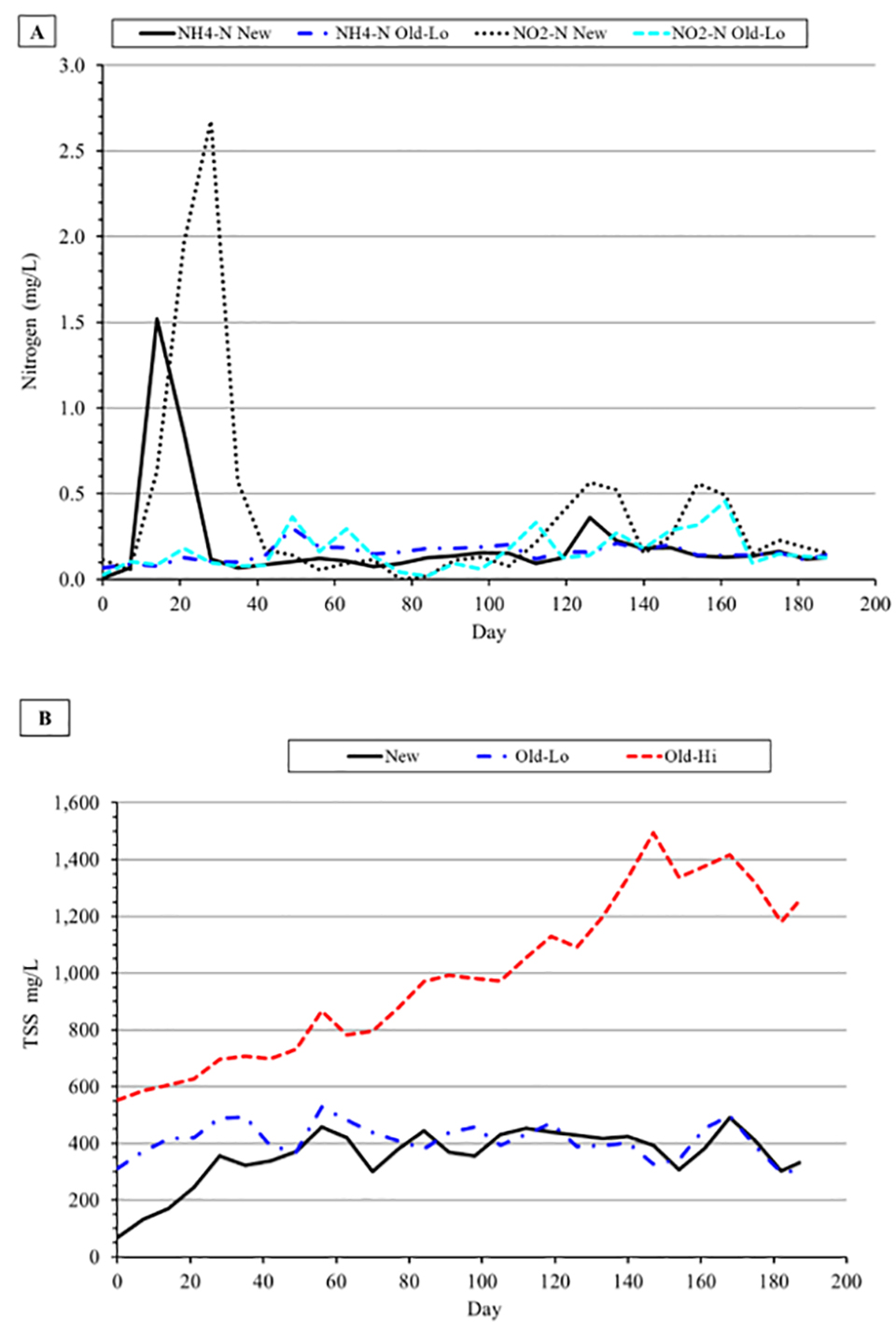
Catfish retain 30 percent of feed phosphorus and in the BFT production system in HDPE-lined tanks excreted dietary phosphorus accumulates in contrast to earthen ponds where phosphorus is adsorbed by bottom soil. Significant differences among treatments in initial mean PO4-P concentration carried throughout the experiment despite similar total feed consumption among treatments. The absence of water discharge from the Old-Hi treatment and the significantly greater discharge of water and solids from the Old-Lo treatment compared to the New treatment likely explain the observed differences. Mean PO4-P concentration was significantly higher in shrimp BFT production system tanks seeded with 50 or 100 percent biofloc water from the previous production cycle compared to tanks that did not receive old biofloc water.
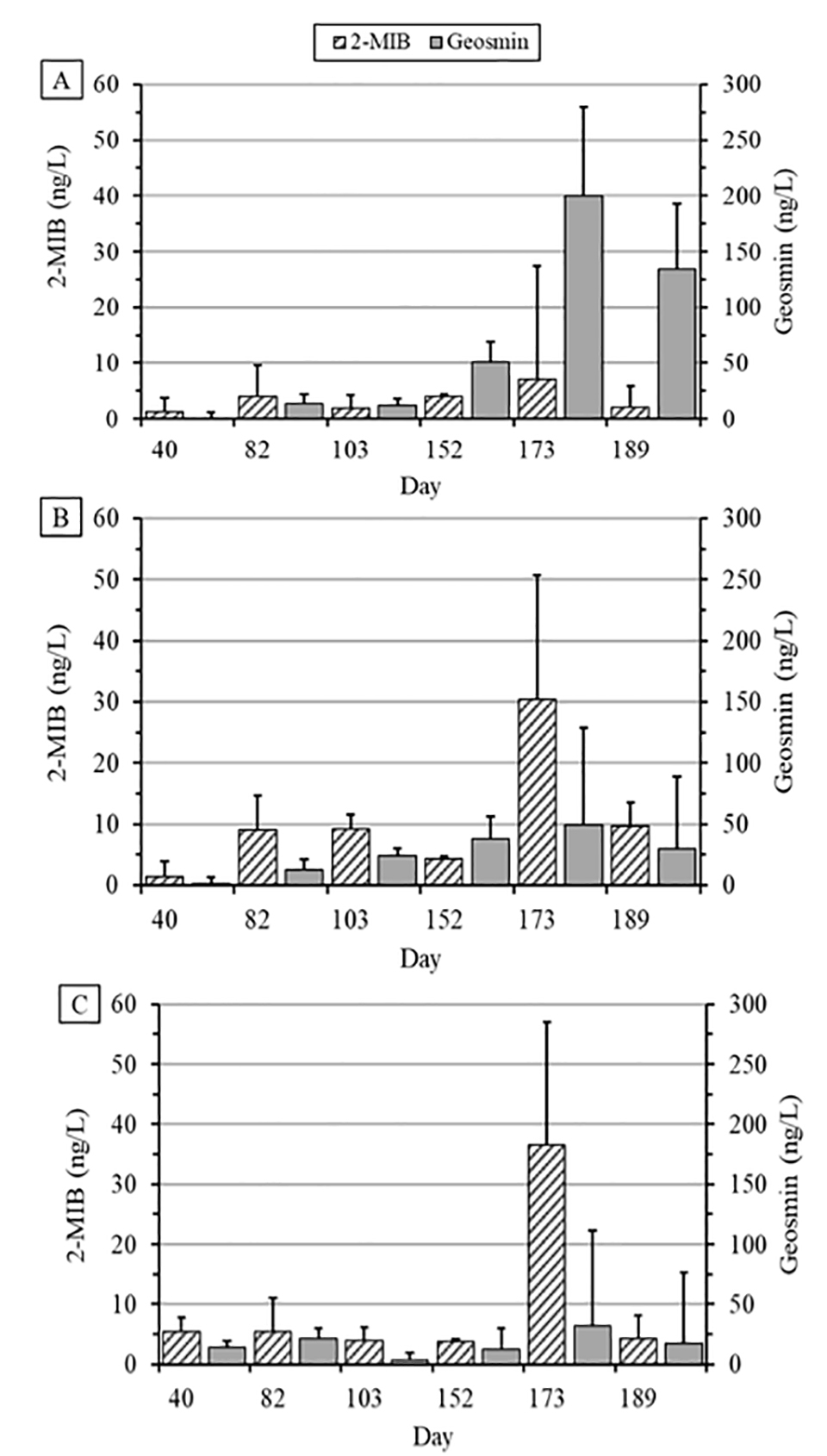
The low concentrations of MIB and geosmin measured in tank water samples on most sample dates except mid-October to early November were consistent with results from other studies that reported production of catfish in the BFT system. The highest MIB concentrations in tank water occurred in one tank each in the Old-Lo and Old-Hi treatments in October and were 84 and 82 ng/L, respectively, well below the typical MIB concentrations that can be present in pond water and result in off-flavor catfish from catfish production ponds in Alabama, Arkansas, Louisiana and Mississippi.
Similarly, the highest geosmin concentrations (472 and 334 ng per liter) were measured in one tank in the New treatment in October and November, respectively. In contrast, significantly higher geosmin concentrations of >2000 ng per liter can occur in the water of catfish production ponds. In another study conducted from July through September approximately 20 to 60 percent of commercial ponds in west Mississippi and 5 to 35 percent of ponds n the Mississippi-Alabama Blackland Prairie had MIB concentrations >100 ng per liter, whereas geosmin concentrations>100 ng per liter were found in 0 to 15 percent and 10 to 25 percent of ponds, respectively. In general, the incidence and severity of these common off-flavor problems are less problematic in BFT systems compared to earthen catfish production ponds in the southeastern United States.
Phytoplankton communities present on the sample dates with the higher MIB and geosmin concentrations were dominated by cyanophytes in all treatments except for the first sample where chlorophytes co-dominated in the New and Old-Lo treatments. However, none of the cyanophyte genera and species found in the present study is known conclusively to produce MIB or geosmin.
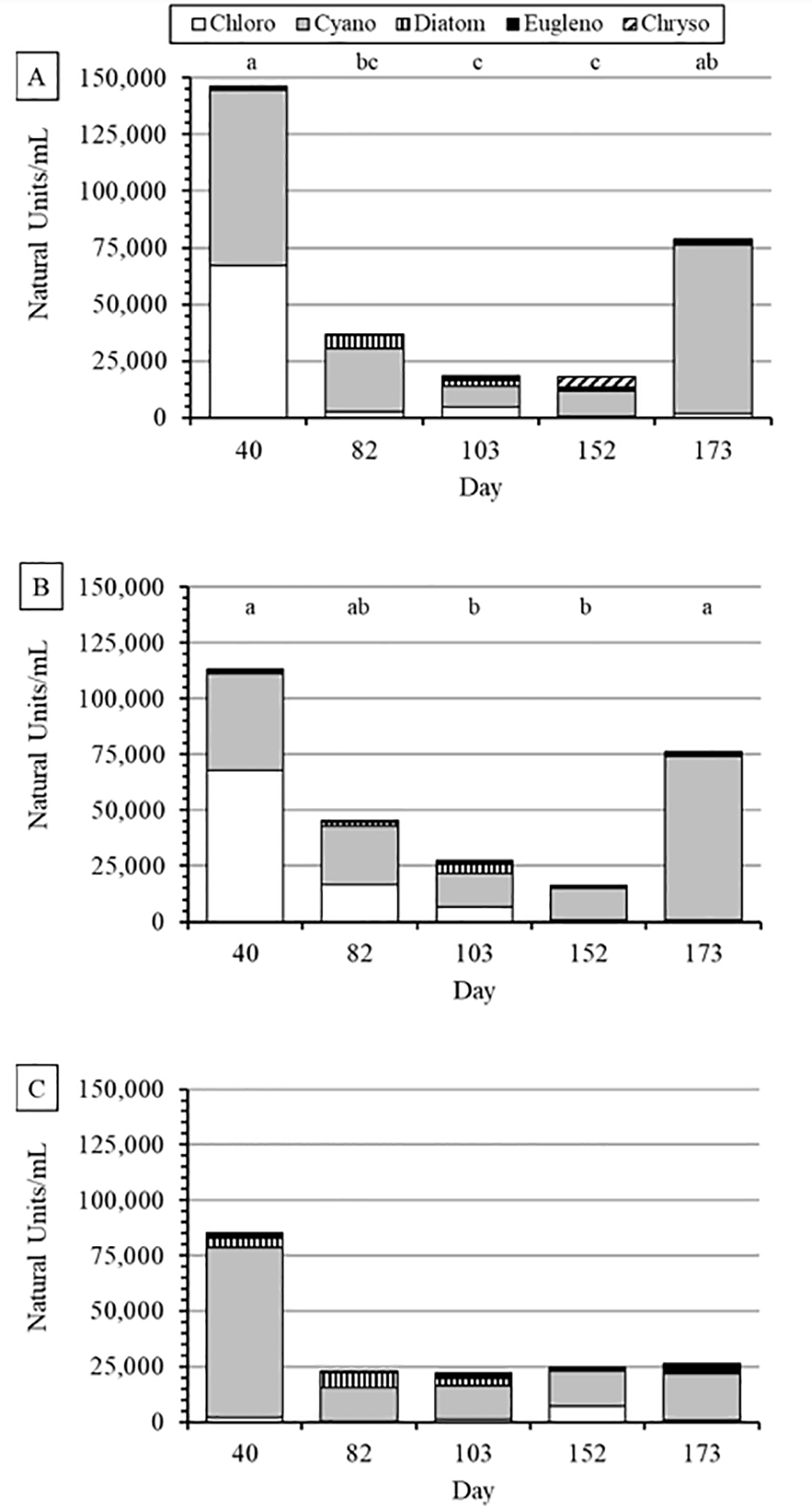
The predominant types of phytoplankton in this study were smaller species of cyanophytes compared to those observed typically in catfish ponds, and they were present usually in close association with the biofloc particles or embedded with the biofloc particles, which may have provided some protection from shear stress and some distribution within the water column from the mixing of the biofloc particles in the water of the BFT tanks.
Whole-body lipid content coupled with water temperature and off-flavor compound concentration are factors that affect the bioaccumulation of MIB and geosmin in catfish flesh. In the present study, the absence of treatment differences in whole-body lipid content does not provide an explanation for the higher geosmin concentrations in the fillets of the New treatment catfish. High mean geosmin concentrations on day 173 and 189 samples suggests that bioaccumulation from prolonged exposure of New treatment fish to geosmin in the culture water may have affected fillet flavor status adversely.
Trained catfish processing plant flavor testers can detect MIB and geosmin concentrations as low as 100 to 200 ng per kg and 250 to 500 ng per kg, respectively. Thus, fish from the New treatment likely would be classified as having an objectionable “earthy” off-flavor. Typically, MIB-related off-flavor is more common than geosmin off-flavor in pond-raised catfish in the southeastern United States, which is in contrast to many of our BFT studies so far. The source of geosmin in tanks where solids were removed is unknown. None of the phytoplankton species found in water samples are known conclusively to produce MIB or geosmin.
Changes over the 181-day study in trace mineral concentrations in water were minimal or below method detection limits. All trace mineral concentrations were below National Recommended Aquatic Life Criteria (USEPA (U.S. Environmental Protection Agency), 1986, 2007, 2019). Treatment differences in final water sample mineral concentrations were related more to TSS removal than to new versus old water. Biofloc particles have a negative surface charge with high affinity for cations, which suggests that solids and water discharge from the New and Old-Lo treatments contributed to the decreased mineral concentration in final water samples compare to the Old-Hi treatment.
As was seen with whole-bodies, fillet mineral concentration of channel catfish grown in biofloc water from the previous year did not differ significantly compared to new biofloc water. Results from the present study suggest that differences in water mineral concentrations among treatments did not affect whole-body or fillet mineral concentrations. However, this study did not evaluate year to year variation in fillet mineral content within location. Mineral concentrations in a 100-g portion (as is) of channel catfish fillet meat were comparable to other published values. Additionally, mineral concentrations in a 100-g portion of channel catfish fillet were within the recommended dietary intake and less than the tolerable upper intake levels.
Perspectives
In summary, one-year old water from a channel catfish BFT production system can be used for a second year of BFT production with no adverse effect on channel catfish production responses. One advantage to re-use of BFT system water is the absence of NH4-N and NO2-N concentration spikes typical of BFT start-up.
Although a maximum TSS concentration of 1415 mg per liter in the Old-Hi treatment did not affect daily feed consumption, variable results from other studies suggest that an optimal upper TSS concentration limit remains to be determined. On the other hand, accumulation of TSS in the channel catfish BFT system, i.e., no TSS removal, appears to favor a lower occurrence of catfish that would be classified as having “musty” or “earthy” off-flavors.
Furthermore, results suggest that TSS removal may reduce nitrification because discharging solids washes nitrifying bacteria out of the system. Macro- and trace-minerals accumulate in water during the culture cycle, and treatment differences were related more to solids removal than to new versus old BFT water. Whole-body and fillet mineral concentrations did not differ when channel catfish were grown in one-year old or new BFT system water.
Further research is needed to determine the number of production cycles for which biofloc water can be re-used and the best strategy and limits for re-use of biofloc water over multiple production cycles.
References available from the original publication.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-

Bartholomew W. Green, Ph.D.
Corresponding author
United States Department of Agriculture
Agricultural Research Service
Harry K. Dupree Stuttgart National Aquaculture Research Center
P.O. Box 1050, Stuttgart, AR 72160-1050 USA[118,111,103,46,97,100,115,117,64,110,101,101,114,103,46,116,114,97,98]
-
Kevin K. Schrader, Ph.D.
United States Department of Agriculture
Agricultural Research Service
Natural Products Utilization Research Unit
National Center for Natural Products Research
P.O. Box 1848, University, MS 38677-8048 USA -
Steven D. Rawles, Ph.D.
United States Department of Agriculture
Agricultural Research Service
Harry K. Dupree Stuttgart National Aquaculture Research Center
P.O. Box 1050, Stuttgart, AR 72160-1050 USA -
Carl D. Webster, Ph.D.
United States Department of Agriculture
Agricultural Research Service
Harry K. Dupree Stuttgart National Aquaculture Research Center
P.O. Box 1050, Stuttgart, AR 72160-1050 USA -
Matthew E. McEntire
United States Department of Agriculture
Agricultural Research Service
Harry K. Dupree Stuttgart National Aquaculture Research Center
P.O. Box 1050, Stuttgart, AR 72160-1050 USA
Tagged With
Related Posts

Health & Welfare
Biofloc technology reduces common off-flavors in channel catfish
In studies that used biofloc systems to culture channel catfish, culture tanks were susceptible to episodes of geosmin and 2-methylisoborneol and subsequent bioaccumulation of off-flavors in catfish flesh.

Health & Welfare
CART regulates food intake in channel catfish
Experiments by the authors showed that the gene activity of the brain neurotransmitter CART changed in catfish in response to feed intake.

Health & Welfare
Genetic response to selection in channel catfish
USDA and partners are developing channel catfish that exhibit superior growth, fillet yield and resistance to enteric septicemia. Although ongoing research continues to select the USDA lines for better performance, studies comparing the strains have been inconclusive.

Health & Welfare
Hybrid, channel catfish show similar immune responses to ich parasite
In a study, the authors evaluated the immune responses and host protection of hybrid catfish and channel catfish against the ich parasite (Ichthyophthirius multifiliis).



