Zero tolerance both problematic, ‘ever-moving’

By 1993, the Joint FAO/WHO Expert Committee on Food Additives concluded a risk assessment concerning the most significant risk of human chloramphenicol exposure, aplastic anemia, as follows:
“The Committee noted the extremely low overall incidence of aplastic anemia and the lack of association between the ophthalmic use of CAP (chloramphenicol) and aplastic anemia. It concluded that human exposure to CAP residues in food of the same order as exposure resulting from systemic uptake after ophthalmic use would not cause any demonstrable alteration in the incidence of the disorder.”
However, the continued adherence to zero tolerance of chloramphenicol residue in food by the European Community indicates that chloramphenicol risks are regarded as real by European regulators, in addition to being a solid and functional trade-protecting instrument. Moreover, zero-tolerance policies expound a clear notion of infringement, which is solely related to illegal veterinary use.
In my recent work on this issue, it has become clear that with regards to chloramphenicol, international trade is dealing with a multisource issue not recognized or understood by governments. This is because Council Regulation EEC No. 2377/90 only deals with one source: actual veterinary use. Any detection of chloramphenicol is regarded as a violation of law, as the detection is only framed in terms of illegitimate veterinary application.
Clinical use of chloramphenicol, other products
Chloramphenicol is still used in human medicine. It has a wide spectrum of activity against Gram-positive and Gram-negative bacteria. Chloramphenicol therapy is usually restricted to serious infections when other drugs are not as effective. In the Netherlands, a number of registered pharmaceutical products incorporate chloramphenicol, mainly in formulations used to treat eye infections. Chloramphenicol is also used intravenously against infections like meningitis.
With regards to the presence of chloramphenicol in the aquatic environment, these human uses beg the question of whether surface waters could be a source for food product contamination. Several pharmaceutical compounds have already been detected in the aquatic environment in Germany.
Focusing on the presence of antibiotics in sewage treatment plant effluents and surface water, a German research group led by R. Hirsch published in 1999 an analysis of water samples for 18 antibiotic substances from the classes of macrolid antibiotics, sulfonamides, penicillins, and tetracyclines. Remarkably, chloramphenicol was detected in the effluent of a sewage plant in southern Germany at a maximal concentration of 0.56 microgram per liter and in surface water at a maximum concentration of 0.06 microgram per liter.
Medicinal products are eventually excreted – metabolized or unmetabolized – by their consumers. The detected presence of chloramphenicol was probably the result of human use and possibly some sporadic veterinary use. However, a large number of ground water samples were taken from agricultural areas in Germany, and contamination with antibiotics was detected at only two sites. Furthermore, manure is not usually disposed of together with municipal wastewater.
This indicated that flux from veterinary applications to the aquatic environment are of negligible importance in Germany, which leaves us with the human applications of chloramphenicol or its natural production by the Streptomycetes.
To give some idea of human chloramphenicol consumption, S. F. Webb estimated the national human consumption of chloramphenicol in the United Kingdom at 377 kilogram per year in 2001.
However, the International Programme on Chemical Safety’s Chemical INCHEM website reported that sales of chloramphenicol are 11-440 times greater in Hong Kong than several western countries and Australia. Environmental contamination of surface water as a result of human use is therefore expected to be much higher in Asia than Germany and other European countries.
When considering other compounds of Annex IV of Council Regulation EEC No. 2377/90, metronidazole is also of interest, as it is used clinically like chloramphenicol, but in much higher quantities. Among other applications, metronidazole is prescribed in cases of protozoal infections. In the U.K., S. F. Webb estimated that about 15.5 metric tons (MT) per year are clinically used. The worst-case predicted environmental concentration of metronidazole was estimated at 2.85 microgram per liter.
When considering the environmental persistence of metronidazole (over a year), the reasonable assumption would be to find metronidazole in surface waters. As with chloramphenicol, routine clinical use of metronidazole could find its way into the food chain via the aquatic environment, and result in another misguided food scare.
Natural sources?
Of the approximately 12,000 antibiotics known, it is the estimated that some 160 are, or have been, used as human medication. Streptomycetes, which account for well over half of these commercially and therapeutically significant antibiotics, are among the most abundant and ubiquitous soil bacteria. Of all the actinomycete isolations from soil, about 90 percent are Streptomycetes. It is therefore no surprise that it is possible to isolate chloramphenicol from Streptomyces venezuelae present in the soil, as stated in the Hazardous Substances Data Bank, an online database produced by the National Library of Medicine.
The question of whether nature itself could be a source of chloramphenicol in food products, apart from the human clinical use, needs to be answered. To that end, the Instituto Technológico Agroalimentario (AINIA) – an accredited nonprofit institution in Spain created by, among others, companies in the food-manufacturing sector – sampled ready-to-sell food products acquired from retailers for the presence of chloramphenicol. Using a commercial enzyme-linked immunosorbent assay (ELISA) kit, the group detected chloramphenicol in numerous products at very low levels.
These results were at best ambiguous, however, as in only one case was chloramphenicol confirmed by high-performance liquid chromatography-mass spectrometry. But the confirmed sample does represent an interesting – albeit unexplainable – caveat for the possibility of a natural bacteriological source of chloramphenicol in the food chain, which merits further research in this area.
A problem with the AINIA data is that at the very low end of the scale, food matrix artifacts to which ELISA responds cannot be differentiated from a real presence of chloramphenicol. The reality of false positives is a well-known problem in the analytical sciences.
Illustrative of this are the results obtained in a recent collaborative trial by U. Schröder in Germany in which shrimp with predefined amounts of chloramphenicol added were tested with blank “unspiked” shrimp. In summary, 50 percent of the participating laboratories designated blank shrimp as positive for chloramphenicol using various analytical techniques – worrying results, given the present political perturbations. Alternatively, they could reflect a natural background level of chloramphenicol, as indicated by the AINIA results. However, any given sample would have been judged positive by these laboratories for chloramphenicol and removed from the market.
Detection limits
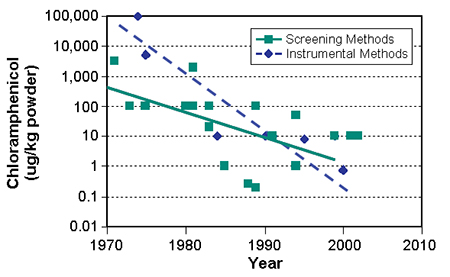
The detection of analytes has improved dramatically during the past decades, including methods used for the detection of chloramphenicol (Fig. 1). For instance, the sensitivity of liquid chromatographic mass spectrometric method equipment has improved tenfold in the last six years, and its fundamental limit has not yet been reached. One can expect that detection of chloramphenicol in parts per trillion will become feasible in the next decade.
Currently there is some confusion about the minimum required performance limits (MPRL) for chemical residues. MRPLs are no more and no less than the concentration levels that regulatory laboratories in the European Community should be able to detect and confirm. The MRPLs should not be mistaken for tolerance limits or any similar terminology.
European Union regulatory laboratories are obligated to try and find residues of banned substances like chloramphenicol at the lowest technically possible concentrations. As a result, and depending on the skills and equipment of laboratories, a concentration lower than MRPL can lead to a positive or “noncompliant sample” result
The E.U. policy of zero tolerance can also lead to economic inequality. Products designated as safe by an exporting country may be designated noncompliant if the importing country uses a more sophisticated method of analysis that results in lower detection limits.
Conclusion
The simple inference espoused by Council Regulation EEC No. 2377/90 – that chloramphenicol detected in food products is solely the result of illicit use in food production – is false. Even if chloramphenicol is sometimes used illicitly in food production, the risk of contracting aplastic anemia as a result of exposure to such dosages is negligible. Any reference to risk as a result of low-level exposure to chloramphenicol is at odds with scientific fact.
Clearly, zero risk is not realistic, and this is even more pertinent when considering the multisource aspect of chloramphenicol, which has never been addressed when reviewing the chloramphenicol issue. The precautionary zero-tolerance solution to illicit use is utterly ineffectual, as no distinction can be made at the low levels dealt with here between environmental contamination, potential natural presence, and fraud.
Considering the vast area of organic geochemistry and secondary metabolisms of numerous organisms, the analytical field is bound to turn up numerous surprises in the future, as limits of detection continue to decrease. More and more chemicals – from indistinguishable sources – will turn up in our food, whereby regulators will add to the confusion of risk. Compliance with zero-tolerance regulation will eventually be unattainable. It will become a legal artifact of the analytical sciences.
This brings us to another flaw of precaution, namely that by the regulatory choice for zero tolerance, the risk of noncompliance is transferred fully to the international market. Zero tolerance is an ever-moving target that requires total compliance of trade partners with no regulatory contribution whatsoever.
In order to circumvent regulatory zero-tolerance instincts, first relevance levels need to be formulated, taking the multisource issue into account. Second, to eliminate differences between exporting and importing countries, consensus has to be reached on the standardization and use of analytical equipment. To achieve these goals, the European Community must liberate itself from the zero-tolerance approach, and the exporting countries must invest in new analytical technology. Within the greater World Trade Organization framework, these must also become goals for both industry and governments.
(Editor’s Note: This article was originally published in the October 2003 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
J.C. Hanekamp, Ph.D.
CEO, The Heidelberg Appeal
Nederland Foundation
P.O. Box 75311
1070 AM Amsterdam
The Netherlands[32,108,110,46,116,101,110,111,114,117,101,64,110,97,104,99,112,97,97,106]
Tagged With
Related Posts

Innovation & Investment
American Unagi brings eel farming back ‘home’
Sara Rademaker launched American Unagi to shift eel farming to American soil, where the eels are from. Why? Because of the novelty, and because she saw an opportunity to do things better.

Health & Welfare
Antibiotic-resistant bacteria, part 1
No antimicrobial agent has been developed specifically for aquaculture applications. However, some antibiotic products used to treat humans or land-based animals have been approved for use at aquaculture facilities.

Health & Welfare
Antibiotics in aquaculture: Is responsible use possible?
Regulations on antibiotics in aquaculture vary by country and region, from outright bans to minimal oversight.
Health & Welfare
ELISA kits offer quantitative analysis of trifluralin in fish
Antibody-based enzyme-linked immunosorbent assay (ELISA) tests are proven, sensitive, high-throughput alternatives to more costly and complex test methods for the detection of herbicide residues and other chemicals.



