In a challenge test, shrimp from breeding program had higher resistance and 30 percent greater survival

Necrotizing hepatopancreatitis (NHP) and its causative bacterial agent NHP-B are widely distributed in several countries in the Western Hemisphere, including the United States and Central and South American countries that farm Pacific white shrimp (Litopenaeus vannamei). This chronic disease causes mortalities of up to 95 percent in shrimp populations in growout and broodstock ponds.
The occurrence of NHP is related to specific environmental conditions, such as high temperatures and high salinities. NHP-B-infected shrimp display a typical soft shell, flaccid bodies, lethargy, reduced feed intake and empty midgut.
Hepatopancreas lesions in the acute phase include intense intracellular hemocytic response, melanized tubules, necrosis and sloughing off of tubule epithelial cells. Hepatopancreas lesions in the chronic phase include marked atrophy of tubules and reduced epithelial cell height, low lipid storage and intratubular edema.
Taura-NHP connection?
In Colombian shrimp culture, NHP is rarely reported in commercial ponds, even when the optimal temperature and salinity for the development of this disease are present. Of special interest is that the presence of NHP-B infections in recent years has been restricted to broodstock ponds or tanks.
When the Colombian breeding program for resistance against Taura syndrome virus (TSV) started in the middle 1990s, most of the broodstock survivors of TSV were also infected with NHP-B. Thus, a possible indirect selection for resistance to NHP could have occurred along with the direct selection for TSV resistance.
Experimental design
To establish if there was some resistance in the Colombian line to NHP-B, a series of experiments were carried out at the University of Arizona Aquaculture Pathology Laboratory during 2008 and 2009. TSV-resistant L. vannamei from Colombia and a specific pathogen-free population from the Oceanic Institute (Kona) were used. The average weight of the shrimp at the start of the test was 2.8 grams. Animals were tagged differentially with fluorescent elastomer tags in their sixth abdominal segments.
Similar quantities from both populations were combined and stocked into two 1-m3 treatment tanks and one 1-cubic-meter control tank. Temperature was adjusted to 30 degrees-C and salinity set to 30 ppt. Shrimp were infected with NHP-B inoculum by reverse gavage. About 100 µL of inoculum was slowly introduced in the anal cavity of each shrimp. Red dye in the inoculum allowed following it first into the hindgut, then into the midgut and hepatopancreas. Shrimp in the control tank were inoculated by reverse gavage using a 0.85 percent saline solution and the red dye.
Shrimp were collected periodically and counted to determine the percentage of survival by population during the testing period. Samples of sick shrimp were fixed in Davidson’s fixative for histological analysis and/or preserved in 95 percent ethanol for polymerase chain reaction (PCR) analysis.
At the end of the experiment, hepatopancreas tissue was taken from surviving shrimp. One-half was frozen at minus-70 degrees-C, and the other half was preserved in 95 percent ethanol. After almost four months, the final survival was determined by counting all the shrimp from both populations present in each tank.
Results
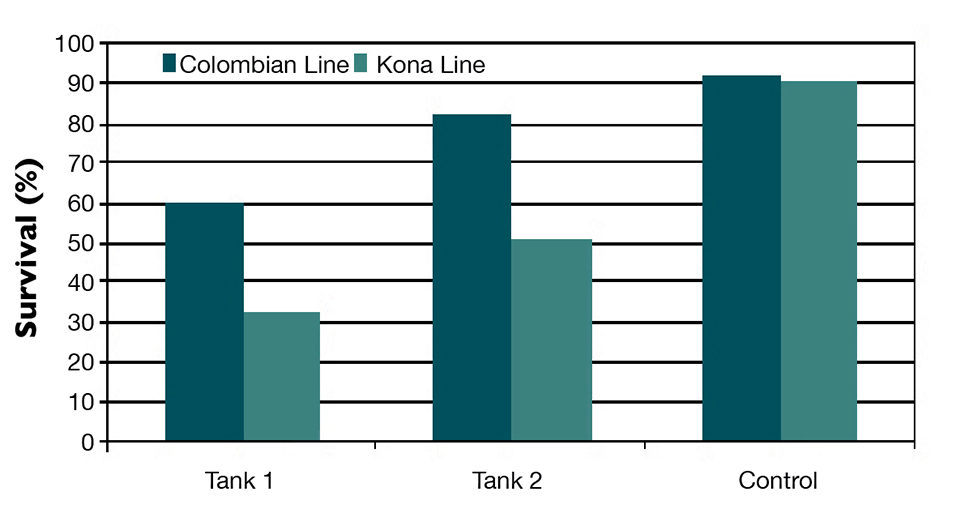
NHP-B infection was confirmed in the infected tanks during the challenge test through histological examination of hepatopancreas tissue and by PCR. However, the pooled survival curve data and final survival of the Colombian population were significantly higher than in the Kona population (P < 0.001).
The Colombian shrimp were clearly more resistant to NHP-B than the Kona population and had about 30 percent greater survival (Fig. 1). The Colombian population’s resistance was consistent with the very low incidence of infection by NHP-B in commercial grow-out ponds in Colombia.
Most of the countries in the Americas that farm L. vannamei have episodes of NHP. Peru, Ecuador, Venezuela, Brazil and most Central American countries have reported NHP outbreaks in juvenile stages that caused mortalities up to 99 percent. Unlike other countries, NHP in Colombia has only been reported in broodstock ponds and just a few cases in grow-out ponds.
The resistance of the Colombian population to NHP-B infection might be related to the process of selection for resistance to TSV. From 1992 to 1995, most Latin American countries that raised L. vannamei were severely affected by Taura syndrome, which caused massive losses in the shrimp industry. Several shrimp-breeding programs were initiated at that time to develop TSV-resistant populations.
Dual selection
In Colombia, the industry set up a mass selection program in which growers selected surviving shrimp from the highly TSV-affected ponds. The most common practice consisted of the transfer of survivors from grow-out ponds at about four months to lower-density ponds before later transfer to the maturation lab. In each transfer, the larger, healthier shrimp with no deformities, flaccid bodies or melanization were transferred to the next phase.
NHP-B had been reported in subadults and broodstock populations in the farms where mass selection was carried out with high prevalence in some ponds. Typical NHP clinical signs in symptomatic shrimp are soft shell and flaccid bodies. Thus, a mass selection program directed to TSV resistance, but which uses as selection criteria survival and elimination of animals with soft shells and flaccid bodies, in the presence of both TSV and NHP-B can simultaneously select for resistance to both TSV and NHP-B. However, TSV selection does not confer a generalized resistance to other pathogens, including NHP-B.
The selection pressure in the initial growout phase may have been low due to low levels of NHP-B in the ponds. However, due to the almost continual presence of NHP-B in the broodstock ponds, uninfected shrimp in the initial selection from grow-out ponds would likely have been challenged by NHP-B in the maturation stage in those ponds. Thus, selection for NHP-B resistance would have probably been enhanced by the continuous exposure to NHP-B, the main disease present in the broodstock ponds.
(Editor’s Note: This article was originally published in the September/October 2010 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Luis Fernando Aranguren
Department of Veterinary and Microbiology
University of Arizona
Tucson, Arizona 85721 USA[117,100,101,46,97,110,111,122,105,114,97,46,108,105,97,109,101,64,117,103,110,97,114,97,102,108]
-
Marcela Salazar
Corporación Centro de Investigación de la Acuacultura de Colombia
Bogotá, Colombia -

Donald V. Lightner, Ph.D.
Department of Veterinary and Microbiology
University of Arizona
Related Posts

Health & Welfare
Updates on shrimp diseases AHPND, NHP at Aquaculture America 2018
Several shrimp-disease papers were presented at Aquaculture America 2018, including one on the detection of AHPND at a site in the United States.
Health & Welfare
New WSSV, TSV genotypes identified in Saudi Arabia
To determine origins of White Spot Syndrome Virus and Taura Syndrome Virus in Saudi Arabia, authors performed genotyping studies and found a new genotype in each of the isolates collected.
Health & Welfare
Study: TSV exposure may lessen YHV effects in white shrimp
Since Taura syndrome virus was in the Americas when yellow head virus become pandemic in Asia, it is possible TSV prevented shrimp in the Western Hemisphere from infection by materials from Asia containing YHV. To test this idea, the authors evaluated the viral interactions in shrimp preinfected with TSV and then challenged with YHV.

Health & Welfare
Biosecurity principles for sustainable production using SPF shrimp
Basic components of biosecurity include knowledge of diseases, adequate detection methods and the use of “clean” shrimp stocks.



