Simple ingredient offers multiple benefits to freshwater fish farmers

Fish farmers know that stress and infections after excessive manipulation or mishandling of their fishes during harvests, transferences, grading, preparation for transport, live hauling, induced spawning and many other routine work activities can cause significant losses of fish. These losses could be minimized by improving handling and preventively using common salt. This article provides some practical information on the routine, preventive use of salt in fish farms.
Why is salt so useful to aquaculturists?
Common salt (sodium chloride – NaCl) is widely available, safe to workers and fish, and leaves no residues on fish flesh, being regarded as safe for use in aquaculture in many countries. Salt is inexpensive and its use is fundamental and often essential in routine handling of freshwater fish. Salt helps to counteract handling stress, restore osmoregulation, prevent and control diseases, improve overall condition and survival of fish prior to and after transportation, helps to alleviate adverse environmental conditions, and supports the well-being of breeder fish during and after spawning activities, among other practical applications and benefits.
Osmoregulation in freshwater fish
For the purpose of this article, osmoregulation in freshwater fish is a physiological process that maintains balanced amount of salts and water in the animal body. Mucus, skin, gills (chloride cells) and kidneys participate in the process, minimizing losses of salts (minerals) and excreting excess water that entered the fish by osmosis. In intensive freshwater aquaculture, fish feed is the main source of minerals to compensate for mineral losses that occur by diffusion through the gills. Thus, the digestive tract plays an important role absorbing dietary minerals and reabsorbing bile salts in an attempt to keep a constant mineral balance in the blood (homeostasis). Gill epithelia, mucus and skin provide physical barriers between environment water and body tissues (cells) and fluids. Hopefully this description will help the reader visualize the great complexity of osmoregulation in freshwater fish. Readers interested in knowing more about osmoregulation should refer to detailed information easy to find on the web, or in a good fish physiology book.
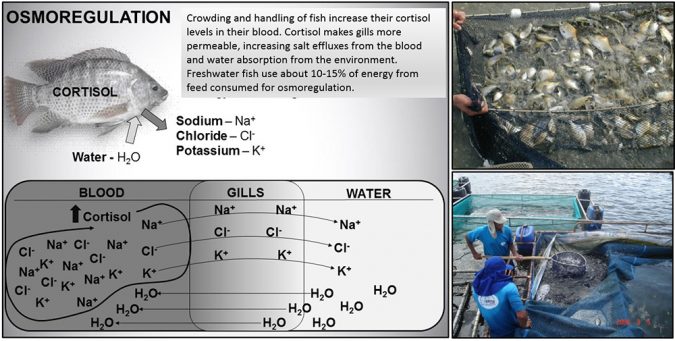
Freshwater fishes keep blood in close contact to the gills. As they live in a diluted (low salt) environment, freshwater fish fight a permanent battle to minimize losses of salt from blood (branchial barriers), to restore eventual losses (through feeds) and to get rid of excess water absorbed from the environment, excreting large volumes of urine. The blood of freshwater fishes contains nearly 9 g of salt per liter, or 0.9 percent or 9 ppt of salt. This matches the blood salt levels of other animals or a saline physiological solution. In general, sodium (Na+) makes up 55 to 75 percent and chlorides (Cl–) another 20 to 40 percent, while potassium (K+), bicarbonate (HCO3–) and other minor ions comprise less than 5 percent of the total blood salts in freshwater fish. Maintaining adequate salt quantities and balance in the blood (osmoregulation) requires a large expenditure of energy by fish and other aquatic animals. By diverting too much energy (feed / nutrients) and attention to osmoregulation, fish may grow slower and neglect other important physiological functions, such as immune defenses.
The salt balance of fish can be adversely affected by physical injuries caused by crowding and handling during harvest, grading, weighing, counting, loading, transporting, artificial spawning and other fish production activities. Extended crowding in nets and tanks triggers hormonal and physiological responses that provoke excessive losses of salts (Figure 1). Gill injuries and inflammation caused by parasites and bacterial infections, suspended solids (clay or organics), and irritating chemicals (such as formalin and potassium permanganate), as well as the frequent exposure to low oxygen, can also make osmoregulation more difficult. Under these adverse situations fish may lose salt to a point of no return. Some species are more tolerant to osmoregulation stress than others. For this reason, farmers should adjust their handling procedures and preventive practices according to the species cultured.
Many uses of salt in fish farming
Most fish farmers are unaware of all the possible applications of salt to reduce fish losses. In fact, salt is often misused or applied too late, and often at very low and ineffective doses and for too short a time. In addition, fish farms commonly lack adequate facilities to handle and treat fish properly when needed. Table 1 includes a list of possible uses for salt in freshwater fish farming. Salt helps to reduce the risk of bacterial and fungi infections after handling, and is an effective and safe product to control some external parasites. Salt treatments may be short in duration and highly concentrated (20 to 50 ppt), or longer and at lower concentrations (12 to 15 ppt). Fish can be normally kept for an indefinite time at physiological salt concentrations (8 to 10 ppt). The addition of salt to water during handling and transport improves fish condition and minimizes mortalities after crowding and handling stress. Adding salt plus gypsum to water is an effective way to prevent the death of breeders in some fish species more susceptible to osmoregulation unbalance after handling and spawning. Salt baths are often used to prevent fungal infections in fish eggs. Salt can also be added to fish feeds, to help fish recover blood salts after handling stress.
| Indication of use | Dose (ppt or g/L) | Time of Exposure |
|---|---|---|
| To restore blood salts and improve condition of fish. In tanks for conditioning (fasting) of fish for transportation. In tanks for handling and grading of fish. In tanks for the recovery of fingerlings after transport. In tanks for the stocking of live fish at processing plants. In tanks and aquaria with live fish for sale. | 3 to 6 | Indefinite. |
| In the transport water. | 5 to 8 | Indefinite during the entire time of transport. |
| To control some gill and skin parasites and flukes. | 50 | Very short immersions (30 seconds to 3 minutes). For severe infections where it will be difficult to repeat the treatment (see more hints along this article). |
| To control some gill and skin parasites and flukes. | 20 to 30 | Short baths (10 to 30 minutes). Often the treatment needs to be repeated two or three times to effectively control some parasites. |
| To control some gill and skin parasites and flukes. | 10 to 15 | Bath of 6 to 12 hours, after which water should be slowly flushed through. It may be necessary to repeat it two or three times. |
| To prevent fungal (Saprolegnia) and external bacterial (Flavobacterium) infections during and after handling. | 3 to 6 | Indefinite. |
| To treat an already established infection of Flavobacterium (fin rot / cotton mouth) and/or Saprolegnia (fungi). | 20 to 30 | Short baths (10 to 30 minutes, depending on fish tolerance). |
| To restore blood salts after stress of crowding, handling and grading for moving fish to other culture units. | Adding salt to feed | 10 to 15 g dissolved in 150 ml of water and evenly poured on 1 kg of extruded pellets fed immediately after transferring fish, and thereafter 2-3 consecutive days. |
| In RAS and biofloc systems to prevent bacterial gill disease, fin rot or fungal infections, as well as to reduce risk of nitrite poisoning. Diseases. | 3 | Indefinite during the entire production time. |
| In static freshwater ponds to prevent nitrite toxicity. | 6 Cl to NO2 | Indefinite. Refer to text for dosing. |
| Fish eggs - preventing fungal and bacterial infections on tilapia eggs. | 4 to 6 | Indefinite. In the egg incubation recirculating system. |
| Fish eggs – control of fungal infection. | 20 to 30 | Short baths (10 to 15 minutes). |
| Prevent losses of brood fish caused by osmoregulation unbalances due to confinement and handling for induced or natural spawning. | 5 to 6 plus gypsum (80 g/m3) | Indefinite, with fish maintained all the time in water with salt and gypsum. After spawning, hold fish at least overnight in this salted water before returning them to ponds next day. |
There are several books, articles and extension sheets on fish diseases, physiology and aquaculture that can help expand the knowledge on the use and benefits of salt in aquaculture. In this article I present some practical situations in which proper salt treatments can help farmers to prevent or minimize fish mortality.
Salt in water where fish is being conditioned (fasting) for live transport
Fish farmers routinely experience mortality of fry and fingerlings after handling stress and transport. Such problems originate right at the harvest and will aggravate after grading and counting or weighing fish to estimate numbers. Fish invariably suffer physical injuries (such as loss of mucus and scales, bruises, punctures, etc.) and lose salt in excess, making it difficult to balance osmoregulation. In addition, the crowding stress and the high-density confinement of fish for depuration (fasting) trigger a sequence of physiological reactions that culminate with the rise of cortisol in the blood (Figure 1). Cortisol is a hormone that increases the permeability of branchial cell membranes, magnifying the loss of salts and the absorption of water by fish. After handling, fish may start to show whitish skin lesions, ulcerative lesions and some fin rot that will progress through the depuration (fasting) and after fish transport. Keeping post-larvae, fry, fingerling and even adult fish in water salted at 3 to 6 ppt will help fish balance osmoregulation. Fish will also produce more mucus in response to salt, which will cover damaged body surfaces, preventing skin and fin lesions from getting worse. Furthermore, concentrations of 3 to 6 ppt of salt inhibit the onset of opportunistic fungi (Saprolegnia) and bacteria (Flavobacterium) infections. All these benefits make common salt a valuable tool that helps farmers improve fish condition, making the animals tolerate and better survive the handling and transportation.
Salt helps to counteract handling stress, restore osmoregulation, prevent and control diseases, improve overall condition and survival of fish prior to and after transportation.
Fasting fish in salted water, however, means no water exchange can be done in the holding tanks. Supplemental aeration is therefore necessary. Some species of fish, such as tilapia and carps, will keep eating their own feces if the fecal material is not flushed out of the depuration tanks. A recirculating system that allows the collection and removal of fecal material using settling basins and mechanical filters will help. In fact, a recirculating system is very useful, since salted water can be maintained in good condition and reused continuously, without the need to discharge it to the environment. If a recirculating system is not available, farmers can place fish inside soft-mesh (5-7 mm) hapas for depuration, making sure that these hapas are suspended away from the tank bottom to prevent fish from eating settled fecal and organic materials (Figure 2).
When transporting freshwater fish, adding salt to the water at 5 to 8 ppt helps minimize the difference in salt concentration between water and fish blood. When fingerlings are transported in plastic bags under optimized fish loads, the total ammonia concentration in transport water normally exceed 40 mg/L. If the fish are not properly fastened, total ammonia may go even beyond 120 mg/L. Fish do not die as the pH of water inside the plastic bags is generally acid, due to the increase in carbon dioxide levels in the transport water. However, high ammonia concentration in water will prevent fish from excreting ammonia by simple diffusion from blood to water. The presence of sodium ions (Na+) in the water favors the active transport of ammonium ion (NH4+) from blood to water, even under the negative ammonium gradient between blood and water commonly observed when transporting fish in plastic bags.

Salt to recover fish and prevent post-transport mortalities
Fish farmers often experience significant fish mortalities in the first two weeks post-delivery of fingerlings. Some of the factors behind these losses include poor preparation of fish for transport, presence of parasites, mishandling at harvest and grading, and badly managed fish transportation. Many fingerling producers do not inspect nor treat the fish to get rid of parasites before shipping. Fish with parasite-infected gills may experience osmoregulation unbalance. In addition, parasites will take advantage of the weakened-stressed fish to rapidly multiply, magnifying the mortality often observed after transport. Also, the fingerlings delivered usually have whitish spots on the skin and fins caused by the onset of external bacteria (generally Flavobacterium columnare) or fungi (Saprolegnia) infections. These spots rapidly progress to more severe lesions, contributing to the increase in fingerling mortalities in the first weeks after transport. An effective way to recover fish from, and minimize losses after, the transport is to receive and hold the fingerlings in tanks with salted water (5 to 6 ppt). I call these “recovery tanks,” where the fish can also be easily treated with formalin or potassium permanganate to eliminate external parasites (Figure 3). If mortality due to bacterial infections is common, fish can even be fed preventively with approved antibiotic, medicated feed after arriving at the farm. In general, four to five days are enough to get the fish fully recovered, with blood salts restored, free of parasites, with necrotic lesions healed, and ready to move to the production units.

Salt to control fungi and external bacteria
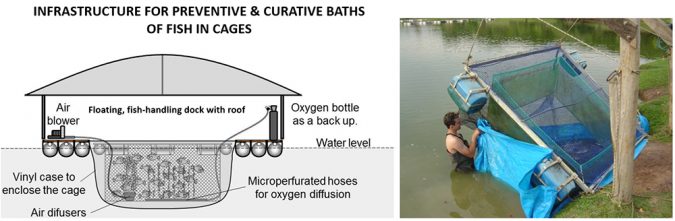
Keeping fish in water with 5 to 6 ppt of salt during handling, fasting and transporting is effective in preventing infections by fungi and external bacterial. However, when the fish are already infected, salt baths of 20 to 30 ppt for 10-30 minutes may be necessary. When farming fish in small volume cages, salt baths can be applied by surrounding the cages with a vinyl enclosure (Figure 4). Aeration is necessary to maintain adequate oxygen levels inside the enclosure during the treatment. Short salt baths (20 to 30 ppt of salt for 10 to 30 minutes) or long baths (10 to 15 ppt for 6 to 12 hours) are the choices.
Applying salt to pond water to treat Saprolegnia or Flavobacterium infection in fish is not as easy and economical compared to the treatment of low volume cages. Other treatment options may be considered for pond fish. For treating a small biomass of high-value fishes (such as ornamental, sportfish and breeders), it is possible to carefully harvest the fish and transfer them to hauling tanks for short treatments. In very small ponds it may be feasible to concentrate the fish with a seine at a pond’s end and delimit a treatment zone of about 10 percent of the pond area by placing a vinyl curtain just behind the seine. The volume of the treatment zone should be estimated and salt applied accordingly to reach at least 10 ppt that should be maintained for 8 to 12 hours. Mechanical aeration is required and dissolved oxygen levels should be monitored frequently for the duration of the treatment. When the treatment is completed, the vinyl curtain and seine are removed, allowing the fish to move out of the treatment zone and the salt to be diluted to the rest of the pond water.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Fernando Kubitza, Ph. D.
Acqua Imagem Serviços Ltda.
Rua Evangelina Soares de Camargo, 115
Jardim Estádio – Jundiai/SP – CEP 13203-560 Brazil
[114,98,46,109,111,99,46,109,101,103,97,109,105,97,117,113,99,97,64,111,100,110,97,110,114,101,102]
Tagged With
Related Posts

Intelligence
Brazilian aquaculture: Constraints and challenges (Part 1)
The Brazilian aquaculture industry has been growing steadily during the last two decades. Despite facing a number of challenges it is looking at continued growth and a larger role in the export markets.

Intelligence
Brazilian aquaculture: Constraints and challenges (Part 2)
Brazil will have to deal with an adverse economic and political environment in the next few years. One should expect high value fish products like shrimp, tilapia, Chilean salmon and cod being replaced by more affordable seafood and alternative meats, as consumers keep losing purchasing power due to inflation, unemployment and monetary devaluation.

Health & Welfare
Proper water circulation in aquaculture ponds critical
Promoting water circulation during the day time is an effective strategy to enrich pond water with oxygen produced by photosynthesis, and can significantly reduce the costs of night time supplemental aeration.

Health & Welfare
Common salt a useful tool in aquaculture, part 2
The preventive use of common salt (sodium chloride) by commercial producers of freshwater fishes has many benefits, including helping with the routine prevention of losses due to diseases, stress and mishandling during transport, harvesting, grading, counting, weighing and induced spawning.



