Prof. Boyd: pH changes seldom result in mortality

Aside from water temperature and dissolved oxygen concentration, pH probably is the most frequently measured water quality variable in aquaculture. These three variables also likely exhibit the most frequent and greatest changes observed among water quality variables in aquaculture systems. Aquaculturists know why water temperature changes and most can give a passable explanation of reasons for changes in dissolved oxygen concentration. Most also understand the consequences of water temperature and dissolved oxygen concentration changes.
However, with respect to pH, most do not have a clue, but they often measure and worry about observed pH changes.
What is pH?
I will begin by reminding readers of the pH concept. The pH is an index of the hydrogen ion (H+) intensity. By definition, pH is the negative logarithm of H+ activity (effective H+ concentration). Hydrogen ion activity and H+ concentration usually are similar, and H+ concentration will be used here. In water at 25 degrees-C and pH 7, there are concentrations of 0.0000001 molar (10-7 M) of both H+ and hydroxyl ion (OH–), and the negative log (pH) of 10-7 M is 7. The reaction of H+ is acidic, while the reaction of OH– is basic (alkaline). It follows that at pH 7, pure water is neutral (neither acidic nor basic). When H+ increases relative to OH–, pH falls below 7 and the lower the pH the greater the acidic reaction of water. When H+ decreases in relation to OH–, the pH increases above 7 and the higher the pH the greater the basic reaction of water.
The pH influences survival and growth of aquaculture animals. The usual effects on aquatic animals of continuous exposure to different pH levels are illustrated in Table 1. At pH around 4 and between 10-11, aquatic animals will die. They usually grow best in the pH range 6.5-8.5, and pH 7-8 likely is optimum for the majority of species.
Boyd, pH changes, Table 1
| pH | Effect |
|---|---|
| 4 | Acid death point |
| 4-5 | No reproduction |
| 5-6 | Slow growth |
| 6-9 | Best growth |
| 9-11 | Slow growth |
| 11 | Alkaline death point |
pH management
This classification of pH effects in Table 1 has been seen by the majority of aquaculturists, and it may well be the reason for much of the preoccupation with pH. In ponds, pH may often be near neutral in the morning and rise above 8.5 in the afternoon. Highly acidic ponds are limed to increase pH, and low pH is seldom seen in culture systems. However, pH in the range of 9-10 are commonplace and especially in the afternoon.
The upshot is that water can be limed to avoid low pH, but high pH for a few hours in the afternoon (or for a few days in some instances) is a characteristic of aquaculture pond waters. Elevated pH surely has some influence on the growth of aquatic animals, but there really is no practical way of consistently avoiding high pH in most ponds. A simple explanation of why pH is different in waters of some areas than in others and of why pH values change rapidly deserves explanation.
The most acidic waters are those that have contacted active acid-sulfate soils. Iron pyrite deposits in such soils oxidize, producing sulfuric acid to cause pH to fall as low as 2 to 3. Such soils are usually in coastal areas – they are particularly common in some coastal areas of Australia and Southeast Asia – but they can be found in other regions. The best alternative is to reject areas with such soils as pond sites. But ponds built on such soils can be made suitable for aquaculture by subjecting them to alternating dry-out periods to oxidize the pyrite and flushing to wash out the resulting acidity. Afterwards, liming can be effective in maintaining pH within an acceptable range.
The most common reason for low pH in ponds are watershed and bottom soils that are highly leached and of low pH as a result of a deficiency of basic cations. Water is low alkalinity and pH usually is 4.5 to 6.5. There are huge areas on all continents with such soils, e.g., much of the coastal plain of the eastern United States. Ponds built on such soils can be limed and limitation of aquaculture by acidic conditions easily overcome. Usually, ponds are limed to increase the alkalinity to around 40 mg/L, and this will raise the minimum pH, which typically occurs in early morning, to 7 or 7.5.
In regions where limestone deposits are common and soils may contain free carbonates, surface waters may have alkalinity concentrations of 50 to 300 mg/L and pH usually is near 8. Arid regions also tend to have surface waters of high alkalinity, and in some cases, pH may be well above 8, even in the morning. Ponds filled from brackish water and marine water sources have alkalinities of 80 to 120 mg/L and pH near 8. Liming usually is not required in waters with alkalinity above around 40 to 50 mg/L.
To summarize, aquaculture ponds should have waters with pH above 6.5 and sufficient alkalinity either naturally or because of liming. The utopian ending of the story would be here, but the story does not end here.
pH fluctuations
The phenomenon of fluctuating pH occurs to a lesser or greater degree in almost all water bodies and is caused by biological processes. In waters with low nutrient concentration (oligotrophic waters), the daily change in pH is small, seldom exceeding 0.5 to 1.0 pH units (Fig. 1). In aquaculture ponds, fertilizers and feeds are applied, and this greatly increases biological activity and much wider pH changes occur over a 24-hour period (Fig. 1).
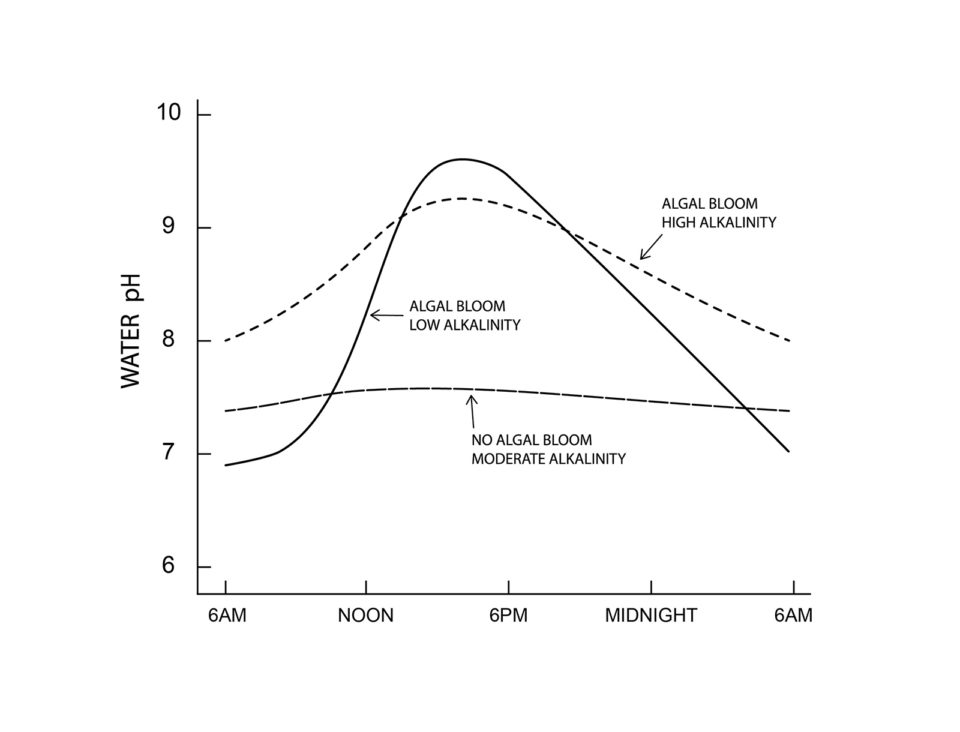
I will spare the reader the complex chemistry of pH change. It will suffice here to say that carbon dioxide is acidic in water. Phytoplankton blooms develop in aquaculture ponds because of the abundant nutrient supply. In the daytime, phytoplankton removes carbon dioxide for use in photosynthesis faster than the respiration of all organisms return carbon dioxide to the water, and pH rises.
There is no carbon dioxide in water of pH 8.3 and above. Many aquatic plants can use carbon from bicarbonate, but this process results in carbonate increasing in the water. Carbonate hydrolyzes with release of OH–, and pH continues to rise sometimes reaching 10 or higher. At night, photosynthesis stops, but respiration continues to return carbon dioxide to the water. The result is that carbonate is transformed back to bicarbonate, pH drops to 8.3, and pH continues to fall in response to increasing carbon dioxide.
Liming of low alkalinity water increases bicarbonate ion concentration to increase alkalinity. Greater alkalinity provides more buffering capacity to minimize pH changes.
Events described above are natural processes and there is no practical means of avoiding them in ponds or other aquaculture production systems that contain phytoplankton. Although pH changes may sometimes negatively affect culture animals, they seldom result in mortality and the effect on production is likely rather minor.
Additional comments
A few additional points about pH are necessary. The pH may rise to 11 or more in ponds with high alkalinity but low calcium hardness. High pH results because the calcium concentration is not sufficient to precipitate enough carbonate (calcium carbonate) from the water to restrict pH from rising above a tolerable level. Fish will most assuredly die within a few hours at pHs above 11. The common way of ameliorating this situation is to apply agricultural gypsum (calcium sulfate) to increase the calcium hardness concentration.
The use of acid-forming fertilizers such as urea or ammonium sulfate in ponds with high alkalinity water and chronically elevated pH has often been recommended as a means of lowering pH. The argument for the use of these fertilizers is that they result in higher ammonia concentration, and oxidation of ammonia by nitrifying bacteria produces H+ to lower pH. While the logic is correct, the amount of fertilizer necessary to have a good result would increase ammonia concentration to toxic levels in high pH ponds.
Sulfuric acid could be applied to lower pH. But, the sulfuric acid neutralizes alkalinity, and the high pH problem would soon return and maybe become worse. Alkalinity in water serves as a pH buffer, and waters with higher alkalinity resist pH change better than those of lower pH. Sulfuric acid treatment lowers alkalinity and this lessens buffering capacity which favors higher pH later on.
The high pH resulting from phytoplankton photosynthesis is more extreme in warm, hot weather in ponds with dense phytoplankton blooms. Some managers have applied algicides to control phytoplankton abundance. Killing a lot of phytoplankton lessens dissolved oxygen from photosynthesis, and the dead phytoplankton decomposes, removing dissolved oxygen sometimes resulting in fish stress or mortality. Moreover, algae blooms will increase again as the algicidal effect declines.
Although pH is easily measured by a variety of means – test strips, test kits, pen-type pH devices and standard electronic meters – the best way to measure pH is with an electronic meter that has been carefully calibrated. The other methods may be considerably in error and the cause of some of the high pH values of concern to aquaculturists.
I hope that this discussion is of some comfort to the chronic pH worriers among you.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
School of Fisheries, Aquaculture and Aquatic Sciences
Auburn University
Auburn, Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Aquafeeds
A look at phospholipids in aquafeeds
Phospholipids are the major constituents of cell membranes and are vital to the normal function of every cell and organ. The inclusion of phospholipids in aquafeeds ensures increased growth, better survival and stress resistance, and prevention of skeletal deformities of larval and juvenile stages of fish and shellfish species.

Responsibility
Appraising pond liners for shrimp culture
The use of plastic-lined ponds by shrimp farmers can significantly improve production efficiency, support more production cycles per year, and higher mechanical aeration rates and stocking densities. The capital cost of lining ponds can be very significant, so a thorough feasibility analysis is recommended when considering this production tool.

Health & Welfare
Ammonia toxicity degrades animal health, growth
Ammonia nitrogen occurs in aquaculture systems as a waste product of protein metabolism by aquatic animals and degradation of organic matter, or in nitrogen fertilizers. Exposure can reduce growth and increase susceptibility to diseases in aquatic species.

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.


