Reviewing the characteristics of various solids and their relevance to key water quality and production parameters

Aquaculture ponds contain a variety of dissolved and suspended solids. Matter is divided into solids, liquids and gases. In aquaculture, water is the main liquid of concern, because it is the solvent in which the solutes (solids and gases) are dissolved or solids are suspended. Gases in water are measured by different procedures than are solids, and they must be treated separately.
Solids, like other types of matter, are made of particles. The most elementary particles are protons, neutrons, electrons, and smaller entities that make up atoms. But, for practical purposes, the term particle is more broadly defined as a small fragment of something (matter), e.g., a piece of organic matter or a speck of soil. Of course, the term also extends to atoms, ions, ion complexes, and molecules dissolved in water as well as to mineral soil particles, living bacteria and plankton, and non-living fragments of organic matter suspended in water. Particles are suspended in water because they settle slowly as a result of low density and small particle size. Turbulence in water also helps maintain them in suspension.
Soluble particles obviously are smaller than suspended particles and consist primarily of atoms, ions, complexed ions and molecules. There is no exact particle size that separates a dissolved particle from a suspended particle. In water quality analysis, particles less than 2 micrometers (mm) or 0.000001 meters in maximum dimension are considered to be dissolved. A water sample is passed through a filter with 2-mm apertures, and the particles passing the filter are dissolved solids while those retained on the filter are suspended solids.
Classification and determination
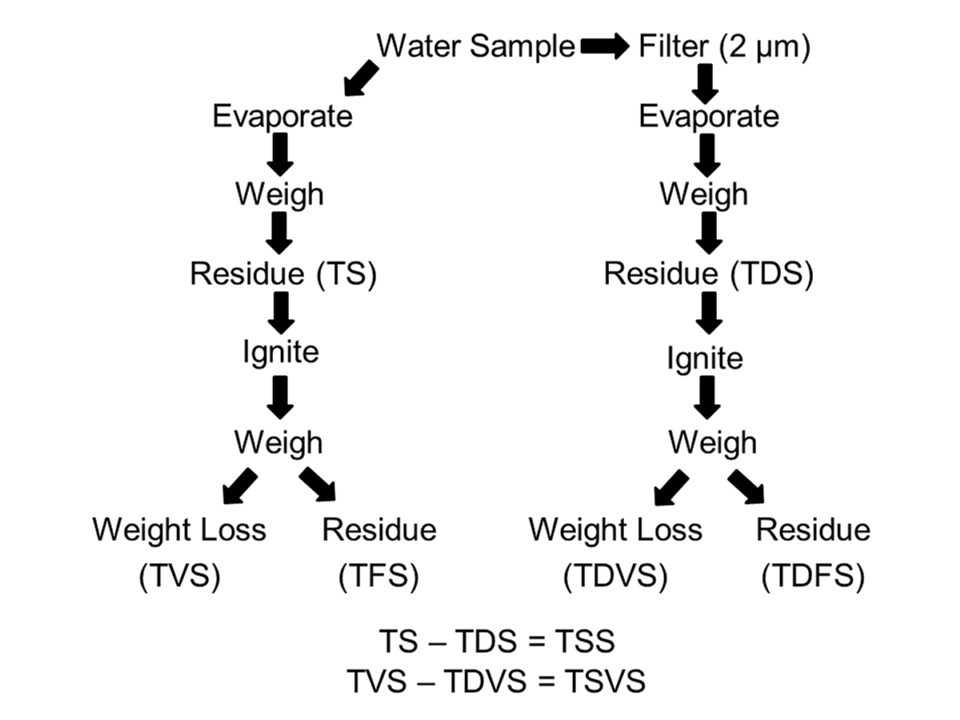
The solids in water are classified by a rather elaborate methodology. The total solids (TS) are determined by evaporating a known volume of unfiltered water in a tared (weighed) dish, determining the weight of the residue and expressing it as weight/volume (usually as milligrams per liter). The total solids include dissolved and suspended solids. The dish and residue from TS analysis can be ignited in a muffle furnace and weighed. The weight loss is called the total volatile solids (TVS) and includes dissolved and particulate organic matter, while the residue is inorganic matter called the total fixed solids (TFS).
The total dissolved solids (TDS) concentration is determined by passing a known volume of water through a 2-μm filter, evaporating the filtrate in a tared dish, determining the weight of the residue, and reporting it in milligrams per liter. The dish and its dry contents can be ignited in a muffle furnace and weighed. The weight loss is the total dissolved volatile solids (TDVS) and the residue is inorganic dissolved solids or total dissolved fixed solids (TDFS). The total suspended solids (TSS) in a sample can be determined as TS minus TDS, and total suspended volatile solids (TSVS) can be determined as TVS minus TDVS.
Solids analysis is a simple but tedious procedure, and the several types of solids make the topic confusing. The method for separating solids is illustrated in Fig. 1 for greater clarity.
In some water quality endeavors, it is only necessary to know the TSS concentration. This can be achieved by passing a known volume of water through a tared, 2-mm glass fiber filter, drying the filter and determining the weight gain. The weight gain is the TSS (or particulate matter) concentration. If desired, the filter and residue may be ignited in a muffle furnace and the weight loss is the TSVS concentration (or particulate organic matter concentration).
The TDS concentration in a water sample usually results primarily from the dissolved ions and mainly from the major cations (Ca2+, Mg2+, K+ and Na+) and the major anions (SO42-, Cl– and HCO3–/CO32-). Salinity is usually a good indicator of the TDS concentration, and conductivity in water increases with increasing concentration of salinity and TDS.
The suspended particles in water tend to be slowly settling in accordance with the Stoke’s law equation. The major factors affecting the settling rate are particle diameter, particle density, and water temperature. Water temperature affects the specific gravity and the viscosity of water. Both of these variables decrease with rising water temperature to cause particles to settle faster.
Planktonic algae range in size from very tiny (0.2 to 2 μm) to fairly large (200 to 20,000 μm). The tiny picoplankton will mostly pass a 2-μm filter and be considered soluble particles. The other planktonic algae are larger and clearly are suspended. Planktonic algae have low densities (1.02 to 1.05 grams per cubic cm). But they are denser than water (1.00 grams per cubic cm) and are subject to settling – especially in water with low turbulence.
https://www.aquaculturealliance.org/advocate/decomposition-organic-matter-impacts-aquaculture-ponds/
Particle settling and sedimentation
A spherical particle will settle faster than particles of other shapes and most planktonic algae are not perfectly spherical. Many are elongated and irregular in shape, some have projections, other form multicellular filaments or colonies, some blue-green algae possess gas vacuoles that increase buoyancy and there are algae with flagella allowing mobility to avoid sinking. Nevertheless, many planktonic organisms ultimately depend upon turbulence to maintain them within the upper layer of water where there is adequate light for photosynthesis.
Other types of suspended solids also tend to settle, but the amount of suspended particles in water is highly dependent upon turbulence. Much feed-based pond aquaculture is done in aerated ponds and aerator-induced water currents both erode soil particles from pond bottoms and help maintain particles in solution. In many aerated, earthen-lined aquaculture ponds, the concentrations of organic and inorganic suspended solids are about equal.
Ponds retain water under relatively quiescent conditions for relatively long periods, and as a consequence, tend to act as settling basins. A recent study of soil cores from about 150 aquaculture ponds of several production intensities in several countries suggested that sediment accumulates in pond bottoms at an average rate of about 1 cm every year. However, the annual rate may be 5 to 10 cm in new ponds and it gradually declines as ponds age and the tendency for erosion declines.
Sedimentation also may occur when waters from aquaculture ponds are discharged into outside water bodies. There is another type of solids measurement for obtaining a quick estimate of the potential of the suspended particles in water to settle. A sample of water is placed in a 1-liter cone that has milliliter calibrations in the narrow, pointed bottom (Fig. 2). This cone is called an Imhoff cone. Water is held in it for 1 hour, and the volume of solids that settle in the pointed bottom is read from the calibrations. This amount of solids (measured in milliliters per liter) is called the settleable solids concentration.
Aquaculture production
Dissolved inorganic solids in water do not affect light penetration. However, dissolved organic solids color water and suspended solids of all types reduce light penetration. Ponds with high concentrations of dissolved organic substances (humic acids) and suspended soil particles often have low rates of phytoplankton photosynthesis. Turbidity from suspended soil particles can be avoided by passing turbid water sources through a sedimentation basin before it enters ponds, installation of practices to lessen erosion on pond watersheds, establishing grass cover on pond banks, lining insides of embankments with plastic sheets and positioning aerators to minimize erosion by aerator-driven water currents.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
School of Fisheries, Aquaculture and Aquatic Sciences
Auburn University
Auburn, Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Responsibility
Aquaculture ponds hold carbon
Although 16.6 million metric tons of carbon are annually buried in aquaculture ponds, estimated carbon emissions for culture species have approached several metric tons of carbon per metric ton of aquaculture product.

Health & Welfare
Dissolved oxygen dynamics
Dissolved oxygen management is the most important requirement of aquaculture pond water quality. DO concentration below 3 mg/L is stressful to shrimp.

Responsibility
Dissolved oxygen management in aquaculture
Management of dissolved oxygen in ponds with static water is difficult, so managers of non-aerated ponds usually limit stocking and feeding rates.

Responsibility
Overview of mechanical pond aeration
Mechanical pond aerators increase the rate at which oxygen from air enters water by providing a greater surface area to facilitate diffusion.

