Enteric septicemia can cause significant damages

Enteric septicemia of catfish (ESC) is the most prevalent disease affecting commercial catfish production. This bacterial disease, caused by Edwardsiella ictaluri, is most prevalent in the fall and spring each year. Clinical signs typically manifest within a few days of exposure and are usually followed by death within two weeks.
In the United States, industrywide losses from ESC outbreaks can be as high as U.S. $60 million in any given year. Current management techniques include vaccination of fry, treatment of infected animals with antibiotics, and withdrawal of feed. However, none of these are completely effective when used alone.
Disease resistance
Resistance to ESC varies among families and strains of channel catfish, but is consistent within each family or strain across multiple challenges. This suggests there may be a genetic component to ESC resistance that could be utilized in a selective-breeding program.
Response to ESC challenge varies widely among NWAC103 strain channel catfish families. Some families show complete resistance to ESC, and others show complete susceptibility. Understanding the mechanisms that drive resistance to ESC will improve efforts to breed channel catfish with enhanced ESC resistance.
Bacterial challenge study
A recent study at the Thad Cochran National Warmwater Aquaculture Center in Stoneville, Mississippi, USA, addressed how bacterial levels and immune response vary relative to the resistance level of multiple families of channel catfish. Six families that showed a consistent response in previous ESC challenges (three resistant, three susceptible) were challenged with virulent E. ictaluri, and bacterial levels and immune response were measured.
A molecular genetic detection assay that enabled rapid, sensitive, and accurate detection of E. ictaluri DNA was utilized to monitor bacterial levels in the blood, kidneys, and spleens of the fish throughout challenge. These tissues are the most likely to be affected during infection.
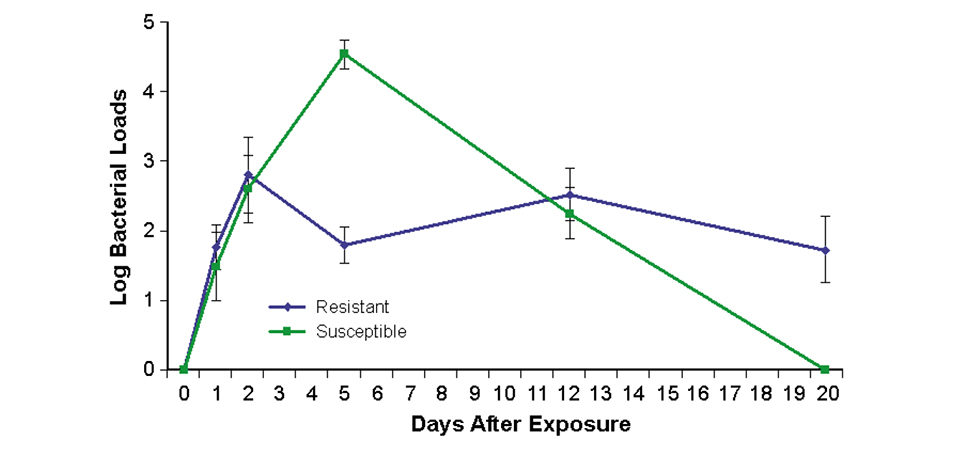
In all cases, bacterial loads were lower in resistant families than susceptible families (Fig. 1). This suggested that bacterial levels were suppressed by some mechanisms of the immune systems in resistant fish.
Immune response
To assess immune response, lysozyme – a nonspecific host response mechanism – was measured. In fish from the resistant families, lysozyme was activated 24 hours earlier than in fish from susceptible families (Fig. 2). This may be sufficient time for bacterial levels to be suppressed.
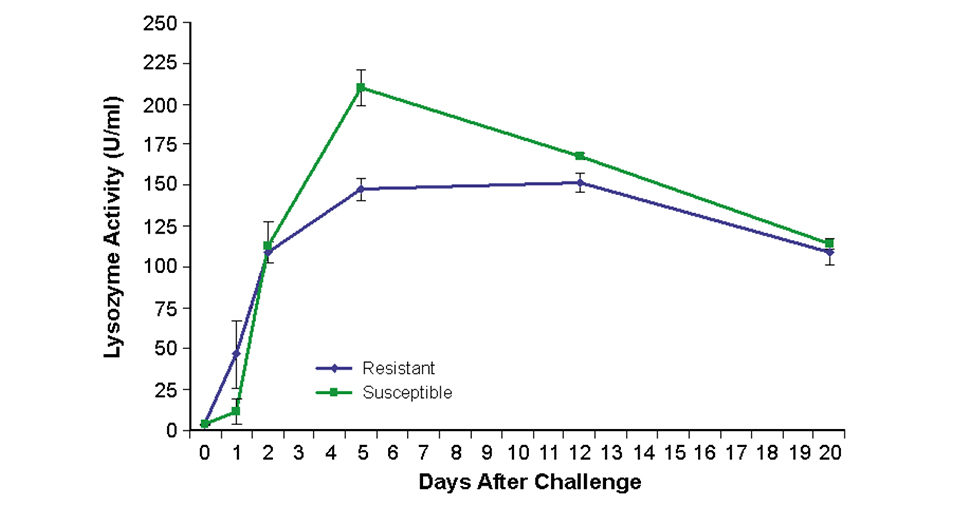
Conversely, the 24-hour delay in response of the susceptible fish may have allowed bacterial levels to increase to a point where acute infection developed and the immune systems could not effectively respond. The low bacterial levels in the surviving susceptible fish may have reflected the ability to maintain moderately low levels of bacteria throughout challenge or pathogen clearance.

Genetic improvement
Studies that address issues related to bacterial loads and immune response provide the framework for building selective-breeding programs for disease resistance. The research described here will be utilized in screening families for innate resistance to ESC, assessment of heritability, development of a germplasm with improved resistance to ESC, and the identification of genes associated with innate immune function and disease resistance for marker-assisted selection.
(Editor’s Note: This article was originally published in the June 2005 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Lelania Bilodeau, Ph.D.
SDA-ARS Catfish Genetics Research Unit
Thad Cochran National Warmwater Aquaculture Center
141 Experiment Station Road
Stoneville, Mississippi 38776 USA
[118,111,103,46,97,100,115,117,46,115,114,97,64,117,97,101,100,111,108,105,98,97]
-
Brian C. Small, Ph.D.
SDA-ARS Catfish Genetics Research Unit
Thad Cochran National Warmwater Aquaculture Center
141 Experiment Station Road
Stoneville, Mississippi 38776 USA
-
William R. Wolters, Ph.D.
SDA-ARS Catfish Genetics Research Unit
Thad Cochran National Warmwater Aquaculture Center
141 Experiment Station Road
Stoneville, Mississippi 38776 USA
-
David J. Wise, Ph.D.
Delta Research Extension Center
Mississippi State University
Thad Cochran National Warmwater Aquaculture Center
Stoneville, Mississippi, USA
Related Posts

Health & Welfare
Antigens provide immunity against ich in channel catfish trials
Vaccination against the Ich parasite is an alternative to chemical treatment. Fish develop a humoral immune response to trophont antigens, with the degree of protection related to the immunizing doses of trophonts used.

Innovation & Investment
Assessing coloration in channel catfish fillets
Because consumers look at color to gauge quality of catfish fillets, the authors developed a digital photography measurement method to assess yellowness.

Health & Welfare
Biofloc technology reduces common off-flavors in channel catfish
In studies that used biofloc systems to culture channel catfish, culture tanks were susceptible to episodes of geosmin and 2-methylisoborneol and subsequent bioaccumulation of off-flavors in catfish flesh.

Health & Welfare
Blue catfish outproduce channel catfish under low-D.O. conditions
Although there is increasing interest in blue catfish, a potential disadvantage of the fish when compared to channel catfish is their reported poorer tolerance of low dissolved-oxygen concentrations.


