Presence of hepatopancreatic microsporidian makes Pacific white shrimp susceptible to AHPND, SHPN

The intracellular, hepatopancreatic microsporidian Enterocytozoon hepatopenaei (EHP) has been reported in cultured black tiger shrimp (Penaeus monodon) and Pacific white shrimp (P. vannamei) in China, Indonesia, Malaysia, Vietnam, Thailand and India, as well as other Southeast Asia countries.
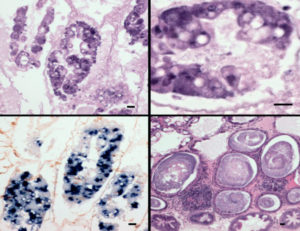
EHP causes growth retardation and increased size variability, and in more advanced stages, infected shrimp have soft shells and exhibit lethargy, reduced feeding and empty midguts. Currently, EHP is diagnosed by histology, In-situ hybridization and PCR.
During 2009–2012, a new, emerging disease called Acute Hepatopancreas Necrosis Disease (AHPND) – also known as Early Mortality Syndrome (EMS) – began causing significant, farmed shrimp mortalities and production losses in most of the countries where EHP has been reported. In some cases, AHPND was also reported in co-infection with EHP. The causative agents of AHPND were determined to be Vibrio bacteria including Vibrio parahaemolyticus, V. campbellii, V. owensii and V. harveyi.
Some Asian shrimp farming countries, especially India and Indonesia, have only been affected by EHP, and so far no cases of AHPND have been reported. These countries have seen an increase of bacterial problems contributing to hepatopancreas Vibriosis, which has been “running mortality syndrome” and “white feces syndrome.” Shrimp affected by these conditions display septic hepatopancreatic necrosis (SHPN).
Because EHP has been affecting the shrimp culture industry before the occurrence of AHPND outbreaks, it is possible that EHP favors the establishment of AHPND and other bacterial diseases, such as SHPN. To determine the relationship of EHP to AHPND and SHPN – supported by the U.S. National Institute of Food and Agriculture under project No. ARZT-5704190-A50-126 – we evaluated the risk factor of EHP with these diseases: AHPND through experimental challenge tests, and SHPN by a case-control analysis. This article summarizes the original publication [Aquaculture 471 (2017) 37–42)].
Study setup
Specific-pathogen-free (SPF) Penaeus vannamei from a commercial facility (Shrimp Improvement Systems in Islamorada, Fla.) were used. The experimental infections were carried out at the Aquaculture Pathology Laboratory (APL), University of Arizona (USA). Please refer to the original publication or contact the first author for a detailed description of the various experiments to propagate EHP infection in SPF shrimp at the APL; AHPND bacterial strains and culture; AHPND pathogenicity bioassays and histopathology; bacterial counts and histopathology in a 12-hour time infection course; the relationship between hepatopancreatic microsporidiosis (HPM) and septic hepatopancreatic necrosis (SHPN) at farm level; histopathology and in situ hybridization (ISH) for EHP; EHP and AHPND PCR assay; and the statistical analyses used in this study.
Results
Regarding EHP infection before AHPND infection, EHP was confirmed by histopathology in samples of EHP pre-infected shrimp and in the EHP-negative control group. Infection severity ranged between G1 and G2 based on a semi-quantitative scale. The infection was more prominent in the medial and proximal regions than in distal region. Before the experimental infection, no AHPND lesions were observed in any animals.
Table 1 shows the results of the experimental VPAHPND infection. No mortality was observed in the negative control tanks in the two experiments. Confirmed by PCR and histopathology, the VPAHPND used in this experiment caused AHPND in the infected animals. The groups exposed to a high dosage of V. parahaemolyticus had high (83 percent and 64 percent) mortalities.
Aranguren, EHP, Table 1
| Experiment | Experimental Group | Treatment | Dosage (CFU/mL water) | No. of dead shrimp/No. total | Mortality (%) |
|---|---|---|---|---|---|
| 1 | AHPND | Low dose | 2.4 X 10 (5th power) | 0/5 | 0 |
| 1 | EHP-AHPND | Low dose | 2.4 X 10 (5th power) | 3/5 | 60 |
| 1 | Positive control | High dose | 2.4 X 10 (6th power) | 5/6 | 83 |
| 1 | Tank control | EHP negative control | 0 | 0/5 | 0 |
| 1 | Tank control | SPF negative control | 0 | 0/5 | 0 |
| 2 | AHPND | Low dose | 2.4 X 10 (5th power) | 2/11 | 18 |
| 2 | EHP-AHPND | Low dose | 2.4 X 10 (5th power) | 4/9 | 44 |
| 2 | Positive control | High dose | 2.4 X 10 (6th power) | 7/11 | 64 |
| 2 | Tank control | EHP negative control | 0 | 0/5 | 0 |
| 2 | Tank control | SPF negative control | 0 | 0/5 | 0 |
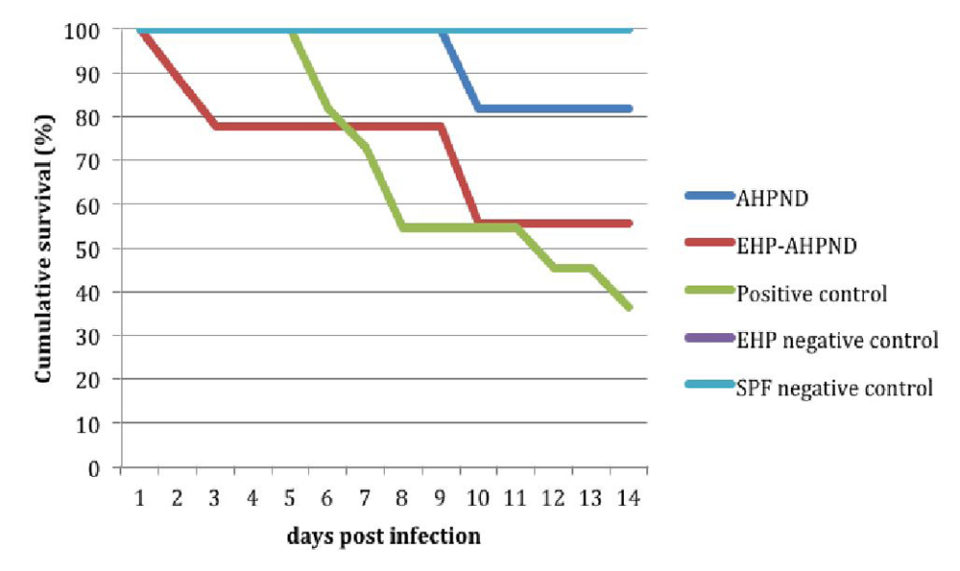
Juveniles from the EHP-AHPND group (shrimp pre-infected by EHP infected with VPAHPND) when challenged with a low dose, had higher mortalities – 60 percent vs. 0 percent and 44 percent vs. 18 percent in experiments No. 1 and No. 2, respectively – than those from the AHPND group (shrimp infected with VPAHPND).
Fig. 1 shows a comparison of the survival curves between the AHPND vs. EHP-AHPND groups was carried out and a significant difference was found (P < 0.05).
Moribund animals examined by histology had lesions typical of AHPND, including a massive sloughing of hepatopancreatic tubule epithelial cells in the medial and proximal regions of the hepatopancreas and progressing outward to the distal region. The terminal phase (characterized by a typical SHPN) was observed in some animals.
Dead animals were analyzed by PCR for AHPND and EHP. In the group AHPND and the positive control tanks, only positive results of AHPND were found. In the group EHP-AHPND, shrimp were positive for both AHPND and EHP. Experiment survivors shrimp did not have any AHPND histopathological lesions.
In the histopathology and bacterial count experiment (12-hour time infection course), no histological lesions of AHPND were observed in the AHPND group during the first 6-hour post-infection (h.p.i); two animals displayed an early phase of AHPND lesions with a severity grade G1 at 12 h.p.i.
In comparison with the ISH, not all the sloughed cells had a positive reaction for EHP, suggesting that the sloughing was present in EHP-infected cells and EHP-uninfected cells. At 12 h.p.i, there was an absence of B cells, the necrosis progressed toward the distal region and a massive sloughing occurred. At 12 h.p.i, 57 percent of the shrimp in the EHP-AHPND group displayed AHPND vs. 11 percent from the AHPND group. No histological signs of AHPND were found in the negative control shrimp.
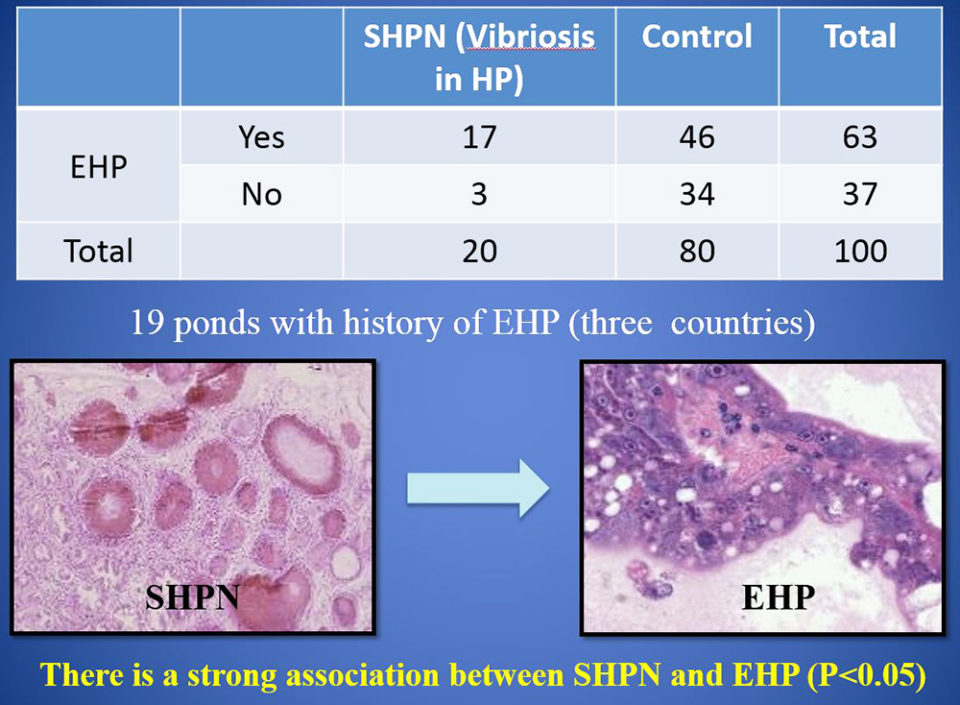
In the case control study (EHP and SHPN at farm level), during 2015-2016, records were analyzed for 100 juvenile P. vannamei shrimp from 19 growout ponds in Southeast Asia farms with history of EHP. Our data show a strong association between cases of SHPN and the EHP-infected shrimp. And the frequency of SHPN was higher in animals with EHP than in those shrimp without EHP.
Perspectives
The strong association found between cases of SHPN and EHP suggests that EHP increases shrimp susceptibility to infection by Vibrio bacteria – known opportunistic pathogens that cause disease when shrimp health is compromised. EHP infection disrupts the cells of the hepatopancreas tubules and allows Vibrio spp. already present to colonize the sloughed cells and the exposed basement membrane.
Using two independent methods, our study showed that EHP-infected Pacific white shrimp were co-infected with AHPND and SHPN. The significant effects of these co-infections are most likely explained by the synergistic actions of the microsporidian and bacterial pathogens on the tissue of the hepatopancreas. Through their combined actions, co-infection of VPAHPND and EHP dramatically increases the damage to the hepatopancreas, to the point of causing total tissue failure and morbidity.
Our results may help explain the observed patterns of emergence of disease outbreaks in shrimp farming countries worldwide. For example, countries in SE Asia – such as China, Vietnam, Thailand and Malaysia – that have suffered EHP outbreaks during the last decade, eventually also had AHPND outbreaks years later. Other countries like Indonesia and India have recently reported the presence of EHP but have not yet experienced outbreaks of AHPND.
However, in Indonesia an emerging disease known as white feces syndrome (WFS) appears to be due to the co-infection of EHP with other opportunistic Vibrio spp. that cause SHPN. Similarly, in India, the running mortality syndrome (RMS) has been recently reported, with affected animals shrimp having severe lesions of the hepatopancreas.
The development of measures to prevent EHP or to treat affected ponds could greatly reduce production losses in shrimp aquaculture, and we encourage this research.
References available from the first author.
Authors
-

Luis Fernando Aranguren, Ph.D.
School of Animal and Comparative Biomedical Sciences
University of Arizona
Aquaculture Pathology Laboratory
Tucson, AZ 85721 USA[117,100,101,46,97,110,111,122,105,114,97,46,108,105,97,109,101,64,117,103,110,97,114,97,102,108]
-

Jee Eun Han, D.V.M. Ph.D.
CJ CheilJedang Feed & Livestock Research Institute
CJ Blossom Park
Suwon-si, Gyeonggi-do, Korea
[109,111,99,46,108,105,97,109,103,64,51,50,50,49,101,106,110,97,104]
-

Kathy F.J. Tang, Ph.D.
School of Animal and Comparative Biomedical Sciences
University of Arizona
Tucson, AZ 85721 USA[117,100,101,46,97,110,111,122,105,114,97,46,108,105,97,109,101,64,117,121,106,103,110,101,102]
Tagged With
Related Posts

Health & Welfare
New management tools for EHP in penaeid shrimp
Authors examined the histological features from shrimp infected with the emerging microsporidian parasite Enterocytozoon hepatopenaei (EHP). A PCR assay method was used to detected in hepatopancreatic tissue, feces and water sampled from infected shrimp tanks, and in some samples of Artemia biomass.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
PCR methods characterize AHPND V.p isolates
In analyzing the plasmid sequence from the whole genome sequences of AHPND V. parahaemolyticus (V.p) isolates, researchers identified a clear geographical variation within the plasmid, and developed PCR methods to characterize AHPND V.p isolates as either Mexico-type or Southeast Asia-type.

Health & Welfare
Four AHPND strains identified on Latin American shrimp farms
Two virulence genes are known to encode a binary photorhabdus insect-related toxin that causes acute hepatopancreatic necrosis disease in shrimp. The pathogenicities of these V. campbellii strains were evaluated through laboratory infection and subsequent histological examination in P. vannamei shrimp.

