Oman lab refines in vitro testing tor tilapia feed

Considerable research has been conducted with tilapia to evaluate cost-effective and regionally available sources of protein for inclusion in tilapia feed. However, fishmeal and soybean meal remain the protein sources of choice for feed manufacturers.
Fishmeal has the correct balance of essential amino acids, is highly palatable and digestible, and has additional properties as an appetite stimulant or attractant. Typical inclusion levels in complete tilapia feeds for use in intensive culture are in the 10-15 percent range of the total diet.
Many alternate protein sources of animal and plant origin continue to be investigated. However, for various reasons, most of them can not completely replace fishmeal. Some ingredients lack sufficient essential nutrients, others contain toxic substances or antinutritional factors, while others reduce palatability.
Measuring digestibility: in vivo methods
Determining the digestibility of protein and other nutrients in feed ingredients is an important step in the formulation of balanced feed. Digestibility, a measure of the quantity of ingested nutrients retained by fish, is most commonly measured by indirect methods using inert marker materials.
By adding an inert material to the feed (external marker) or measuring an inert natural component of the food (internal marker), the apparent digestibility (AD) can be calculated by comparing the ratio of the marker in the food and feces to a specific nutrient. In the formula for determining AD below, the term apparent digestibility is used since feces are contaminated with endogenous material, and hence the values are not “true” values.
AD ( percent) = [1 – (F/D x Dm/Fm)] x 100
Where AD = apparent digestibility
F = percent nutrient or energy in feces
D = percent nutrient or energy in diet
Dm = percent marker in diet
Fm = percent marker in feces.
To be effective, markers must be indigestible, nontoxic, and completely inert. They should move through the gut at the same rate as the digesta.
By far the most commonly used external marker in digestibility studies, chromic oxide is also widely used in tilapia studies. Some results, however, question its validity with tilapia. There is some evidence that chromic oxide can partially separate from food during ingestion and passage through the gut, and that it may not be totally inert. Previous reports from the authors’ laboratory suggest the use of acid-insoluble ash as an alternative marker for tilapia studies.
Digestibility values for individual ingredients are determined by substitution of the test ingredient in a reference diet. Digestibility values for both reference and test diets are measured and values substituted in the following formula:
AD of test ingredient = AD of test diet
– 0.7 AD of reference diet/0.3 AD of test diet
There is little standardization of methodology for feeding and the collection of fecal material during in vivo digestibility studies. Rapid separation of feces from the water is essential to reduce the contamination, breakup, or loss of soluble materials. This is generally performed using a trap or conical tank from which feces can be removed periodically through a collection arm.
Measuring digestibility: in vitro methods
The published digestibility values of ingredients used to formulate tilapia feed were obtained using the in vivo methods outlined above. Measurement of ingredient digestibility using in vitro methods may provide an additional measure of the biological value of proteins for use in aquafeed. Various approaches have been taken, including the use of single or multiple enzyme treatments to simulate peptic digestion, monitoring pH changes, and maintenance of pH using pH-stat methods to record the hydrolysis of peptide bonds.
Current studies in the College of Agricultural and Marine Sciences at Sultan Qaboos University in Muscat, Oman, focus on the measurement of free amino groups released by tilapia enzymes from ingredient samples. Modified from in vitro digestibility studies with salmonids, the study method has been used to compare fishmeal and fish hydrolysates prepared from fisheries by-catch for use in tilapia feed.
In this method, enzymes are extracted from homogenized proximal intestine and separated by centrifugation and dialysis. Chymotrypsin is then assayed against an N-Succinyl-LAla-LAla-LPro-LPhe-pNitroanilide substrate to determine activity levels.
The required 1 DA per minute enzyme volume is added to ingredient samples, which are stirred and incubated at 25 degrees-C for 18 hours. Samples of 1 ml are then cooled and centrifuged. Trinitrobenzensulphonic acid (TNBS) at 0.1 percent is added to each sample, which is then incubated for one hour at 60 degrees-C. When the samples cool, the reaction is stopped by the addition of acid and absorbance measured at 420 nm.
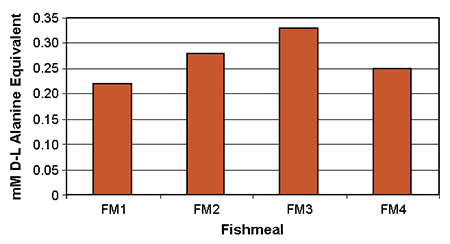
The TNBS measurements are transformed into alanine equivalents from a DL alanine standard curve. As an example, the values of enzyme-released, free amino groups from four fishmeal samples are shown in Fig. 1.
The test detects differences in the quantity of amino acids released by enzymes from protein sources. The initial results obtained from in vitro measurements were closely correlated with the amino acid profiles of the tested fishmeal and results obtained from tilapia growth experiments.
Conclusion
Test results confirmed that in vitro enzyme analyses can be utilized for the assessment of the nutritive value of aquafeed ingredients. Researchers are now extending their work to include a wider range of protein sources and the use of more purified and optimized enzyme fractions.
(Editor’s Note: This article was originally published in the December 2003 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Stephen Goddard, Ph.D.
Department of Marine Science and Fisheries
College of Agricultural and Marine Sciences
Sultan Qaboos University
P.O. Box 34, Al-Khod 123
Muscat, Oman[109,111,46,117,100,101,46,117,113,115,64,100,114,97,100,100,111,103,115]
-
Saiyed Ahmed, Ph.D.
Department of Marine Science and Fisheries
College of Agricultural and Marine Sciences
Sultan Qaboos University
P.O. Box 34, Al-Khod 123
Muscat, Oman -
Ghazi Al-Shagaa
Department of Marine Science and Fisheries
College of Agricultural and Marine Sciences
Sultan Qaboos University
P.O. Box 34, Al-Khod 123
Muscat, Oman
Tagged With
Related Posts

Innovation & Investment
Aquaculture Exchange: Daniel Benetti
University of Miami professor says the U.S. seafood marketplace needs to embrace 'plate-sized' fish if a domestic aquaculture industry is to become sustainable and profitable.

Aquafeeds
Digestibility of fishery byproducts tested
A study of shrimp feeding demonstrated the digestibility of byproducts prepared from salmon livers, salmon milt, black cod viscera and arrowtooth heads and viscera from Alaskan fisheries processing plants.
Aquafeeds
Extrusion processing of aquatic feeds, Part 1
Depending on the extrusion processing conditions and ingredients used, different products such as floating or sinking feed can be produced.

Innovation & Investment
Global Aquaculture Innovation Award 2019 finalist: Arbiom
Global Aquaculture Innovation Award finalist Arbiom is steadily proving the efficacy of its alternative feed ingredient SylPro, made from woody biomass.


