A look inside the University of Arizona’s iconic West Campus Agriculture Center research facility

Since the inception of the University of Arizona’s Aquaculture Pathology Laboratory (APL) and West Campus Agriculture Center (WCAC) in 1989, the research facility has been an integral part of this iconic laboratory.
The APL-WCAC research facility is located more than 200 miles from the nearest ocean, which has proven to be the ideal place to study penaeid shrimp and other crustacean pathogens without risk of introduction into their natural habitat. Over the past 28 years, APL-WCAC has helped to develop pathogen resistant stocks for Pacific white (Penaeus vannamei) shrimp breeding program of the U.S. Marine Shrimp Farming Consortium. It has also developed challenge methods for multiple pathogens which aid in the selection of genetically superior stocks, tested numerous products against viral and bacterial pathogens, run primary quarantine to aid in the diversity of the gene pool in captive breeding, and studied new and emerging pathogens in live shrimp.
APL-WCAC has also trained graduate students, participated in research that resulted in numerous publications, and aided in training of visiting professionals. In the coming years, APL-WCAC will provide support to the global shrimp industry in developing therapies against viral, bacterial and fungal diseases, assist in captive breeding programs and in developing detection tools for emerging pathogens.
Here we provide an overview of the accomplishments and future of the research facility from 1990 to 2015 under the directorship of Dr. Donald V. Lightner, to his successor in 2017, Dr. Arun K. Dhar.
Aquaculture pathology facilities and staff
APL is located on both the main and west campuses of the University of Arizona in Tucson, Arizona, USA. APL has diagnostic and research laboratories stationed on the main campus, and wet-lab facilities at the WCAC, where live animal research has been performed since 1990. Previously, the facility was positioned at a different location leased by the university and was much smaller in size. Although a few studies were completed at this older location, the move to WCAC allowed studies to be performed in both larger scale and with greater frequency.
The APL-WCAC facility includes five independent modular wet-lab buildings, a building that serves as both storage and a workshop, and a building that has an office and a laboratory space. The first building exclusively houses the Specific Pathogen Free (SPF) Penaeus vannamei used in most studies performed at APL-WCAC and the main campus.
The SPF building contains 14 1,600-liter fiberglass tanks that hold certified SPF shrimp used in most live shrimp studies at APL-WCAC (Fig. 1). This building is also where the biological filtration is acclimated for use. This biological filtration is used in all studies run at APL-WCAC, is easily adjusted for the needs of each study, and discarded after use to guard against cross-contamination.
Another wet-lab building is used solely for primary quarantine of stocks. The remaining wet-lab buildings are for research and hold tanks of various sizes utilized for studies requested asked by clients from around the world. Each of these buildings have characteristics that can be useful for a variety of challenges. For example, one building contains six rooms with a greater range of temperature control while other buildings have greater flexibility for tank placement and tank sizes.

Three sizes of tanks are primarily used in the work performed at APL-WCAC: large fiberglass tanks (1,000-1,600 liters) and two sizes of glass aquaria (90 liters and 37 liters). Twelve 1,000-liter to 1,600-liter round fiberglass tanks are used solely for holding primary quarantine stocks (Fig. 2). An additional 38 1,000- to 1,600-liter fiberglass tanks are used in research projects or for running large-scale challenges with shrimp ranging in size from post larvae to adults.

A total of 45 90-liter glass aquaria are available and are utilized in challenges with less biomass and for studies with varying number of replicates (Fig. 3). Six bank systems, each housing up to 24 37-liter glass aquaria are utilized when a larger number of tanks is required. Each bank system is set up as a recirculating system and two banks are housed in three different rooms, allowing APL-WCAC to run statistically significant studies with up to 48 tanks per study (Fig. 4).

The laboratory at the WCAC is equipped with a variety of instruments to assist in the live shrimp challenges performed. This laboratory is used to prepare tissues and inoculums for challenge work, prepare samples for submission to the main APL-WCAC laboratory for diagnostic work following a client study, produce pelleted diets for client studies, and preparation of products needed in a variety of bioassays.

The current staff at the APL-WCAC shrimp research facility is comprised three, full-time staff members. Co-author Brenda Noble joined the APL-WCAC in 1991 and serves as the manager of the facility. She helps university personnel, students and clients develop studies and is responsible for overseeing the facility and staff at the research facility.
First author and assistant manager Paul Schofield joined in 1995. Schofield specializes in producing medicated feeds for clients, designing aquaculture systems, overall care of the animals and is responsible for purchasing and procurement for the APL-WCAC.
Co-author Tanner Padilla joined the APL-WCAC group in 2012 and is responsible for facility maintenance, all aspects of conducting studies, documentation, and monitoring of health of stocks. Co-author Dr. Arun K. Dhar oversees the management of the operation, study design, client visits and follow-up collaborations with researchers from industry and academia alike.
Studies involving shrimp and other aquaculture species
Numerous marine shrimp species have been successfully utilized in research projects and challenge studies at the APL-WCAC. The most common are P. vannamei and P. monodon, but have also included P. stylirostris, P. setiferus, P. duorarum, Marsupenaeus japonicus, Fenneropenaeus merguiensis, F. chinensis, Farfantepenaeus aztecus, Fa. duorarum and Sicyonia ingentis. Freshwater shrimp challenged at APL-WCAC include Palaemonetes pugio, P. paludosus and Macrobrachium rosenbergii. Recently disease challenge studies have also been conducted for different species of oyster that include Crassostrea gigas, C. virginica, C. sikamea and Ostrea lurida.
Pathogen challenge studies using shrimp
The APL-WCAC has challenge methods for Taura syndrome virus (TSV), white spot syndrome virus (WSSV), acute hepatopancreatic necrosis disease (AHPND), infectious hypodermal and hematopoietic necrosis virus (IHHNV), yellow head virus (YHV), infectious myonecrosis virus (IMNV), Vibrio sp., and Enterocytozoon penaei (EHP).
APL researchers have developed standardized challenge protocols for some of these pathogens and utilizes modifies other established challenge protocols to suit specific requirement of an on-going experiment for other major shrimp diseases. In working with the shrimp farming industry since 1995, the APL has helped to develop and confirm the resistance of family lines to TSV and WSSV.
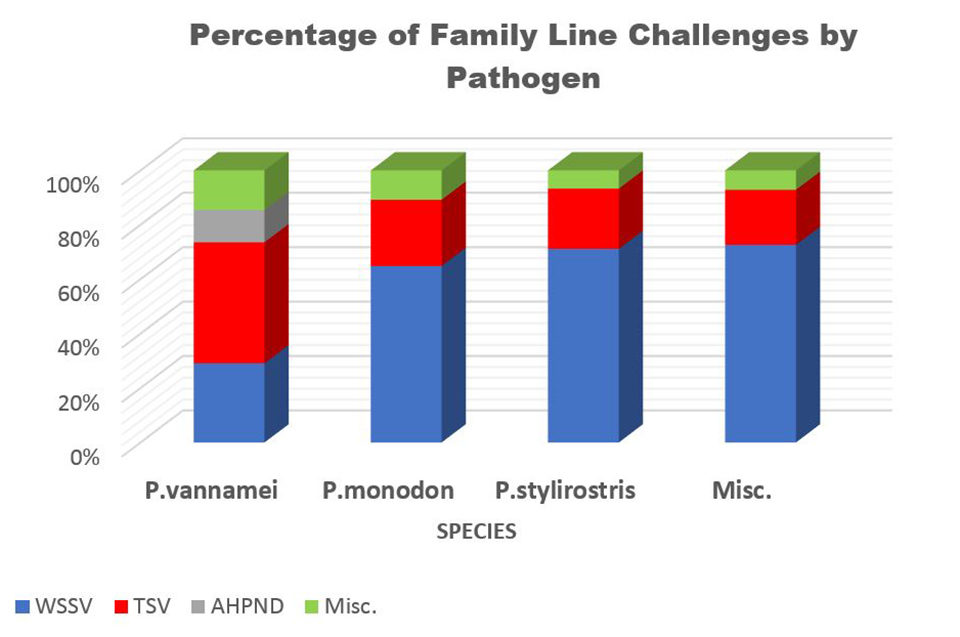
Family challenges comprise one of the largest requests by the aquaculture industry of our research team. Over the past 22 years, a total of 2,383 families have been challenged with one of our TSV isolates (Hawaii, Thailand, Belize, and Mexico). A total of 1,676 families have been challenged with WSSV since 1999 and 544 families have been challenged with IMNV since 2004. Additionally, there have been 624 families challenged with AHPND since 2013 and 157 families challenged with YHV since 1995 (Fig. 5).
Pathogen challenge studies with shellfish and fish
Although the bulk of the challenges performed at the APL-WCAC are with penaeid shrimp, various challenges have been conducted with other aquaculture species. There have been WSSV challenges with the crayfish Cherax quadricarinatus, Procambarus acutus, P. clarkii and Orconectes virilis as well as the American lobster (Homarus americanus) and the blue crab (Callinectes sapidus). TSV challenges have been performed with Cherax quadricarinatus, C. tenumimanus and Macrobrachium rosenbergii. Some fish species have also been challenged in the past, including Oreochromis sp, Pomacnathidae angelfish and freshwater Poecilia sphenops. More recently, stocks of oysters were challenged with Ostreid herpesvirus 1 µvars (OsHV-1 μvar), an emerging pathogen of oyster worldwide to determine the susceptibility of different oyster species to OsHV-1 μvar.
Studies to evaluate feed and feed additives
As the shrimp industry evolved from a subsistence level of farming into a global industry, diseases continued to pose a serious threat for sustainable growth of the industry. Efforts were made to identify etiologic agents, identify tools for detecting those pathogens and strengthen biosecurity measures to contain diseases. Despite preventative measures, disease epizootics continued to occur periodically. This highlighted the need to develop therapies against viral, bacterial, and fungal diseases
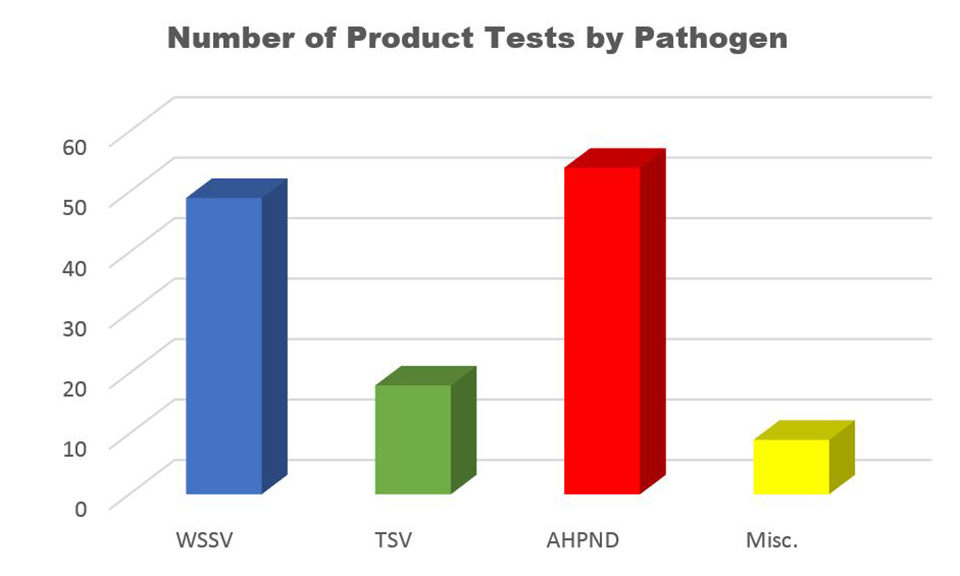
Since 2002, APL-WCAC has worked with companies from around the world to evaluate the efficacy of various products against TSV, WSSV, AHPND and other pathogens. The products tested include antivirals, immunity enhancers, pre-biotics and probiotics, filtering methods, vaccines, immunostimulants, vitamins, algae and yeasts. The application of these products has varied greatly, with some being added to feed (oral delivery with medicated feed being produced either at APL-WCAC or provided by the client), injected directly into the shrimp (injection delivery), or added into the water in a tank containing shrimp (delivery via immersion) (Fig. 6).
Quarantine studies
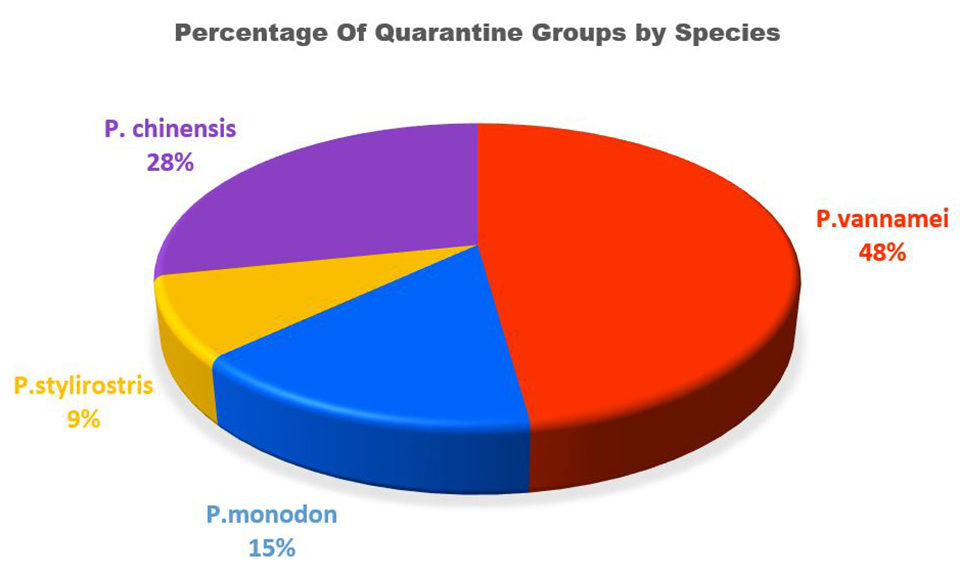
Since 1994, primary quarantining of shrimp has been a large part of the work performed at the APL-WCAC. The University of Arizona has screened 59 groups of penaeid shrimp for primary quarantine. These stocks are held a minimum of eight weeks and are screened for the major World Organisation for Animal Health (OIE) pathogens and any known lesser pathogens along with any emerging pathogens. These quarantines have helped to maintain genetic diversity in breeding as well as aided in the development of pathogen resistant stocks (Fig. 7).
The future of the APL
The University of Arizona’s Aquaculture Pathology Laboratory continues to work with a variety of clients to help in the advancement of aquaculture throughout the world. The focus of the entire team is to provide very high-quality services in developing disease challenge methods in an ever-evolving world of challenge methodology and disease emergence, effectively evaluate therapeutics against diseases in shrimp, screening and identifying genetically superior lines of animals for diseases, growth and other quantitative traits and providing quarantine services to enhancing genetic diversity in the gene pool of captive breeding program.
The University of Arizona is currently collaborating with other universities and private companies on a variety of projects including ways to develop and improve the challenge methods for both EHP and AHPND. Additionally, under the new directorship of Dr. Dhar, the university is expanding its capabilities into genomics research to meet the emerging needs of the global shrimp industry.
Authors
-

Paul J. Schofield
Research Specialist
The University of Arizona
West Campus Agriculture Center
Tucson, AZ 85705 USA[117,100,101,46,97,110,111,122,105,114,97,46,103,97,64,102,111,104,99,83,80]
-

Brenda L. Noble
Research Specialist, Sr.
The University of Arizona
West Campus Agriculture Center
Tucson, AZ 85705 USA[117,100,101,46,97,110,111,122,105,114,97,46,103,97,64,101,116,105,104,119,98]
-

Tanner J. Padilla
Research Aide
The University of Arizona
West Campus Agriculture Center
Tucson, AZ 85705 USA[117,100,101,46,97,110,111,122,105,114,97,46,108,105,97,109,101,64,97,108,108,105,100,97,112,116]
-

Arun K. Dhar, Ph.D.
Associate Professor
Director, Aquaculture Pathology Laboratory
The University of Arizona
West Campus Agriculture Center
Tucson, AZ 85705 USA[117,100,101,46,97,110,111,122,105,114,97,46,108,105,97,109,101,64,114,97,104,100,97]
Tagged With
Related Posts

Health & Welfare
Big shoes to fill: Dhar takes reins at shrimp pathology laboratory
Arun Dhar, Ph.D. will attempt to fill the “big shoes” of Dr. Donald Lightner at the University of Arizona’s Aquaculture Pathology Laboratory, where the shrimp disease EMS was diagnosed.

Health & Welfare
Four AHPND strains identified on Latin American shrimp farms
Two virulence genes are known to encode a binary photorhabdus insect-related toxin that causes acute hepatopancreatic necrosis disease in shrimp. The pathogenicities of these V. campbellii strains were evaluated through laboratory infection and subsequent histological examination in P. vannamei shrimp.

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

