Learn to identify normal and abnormal cellular elements present in the blood
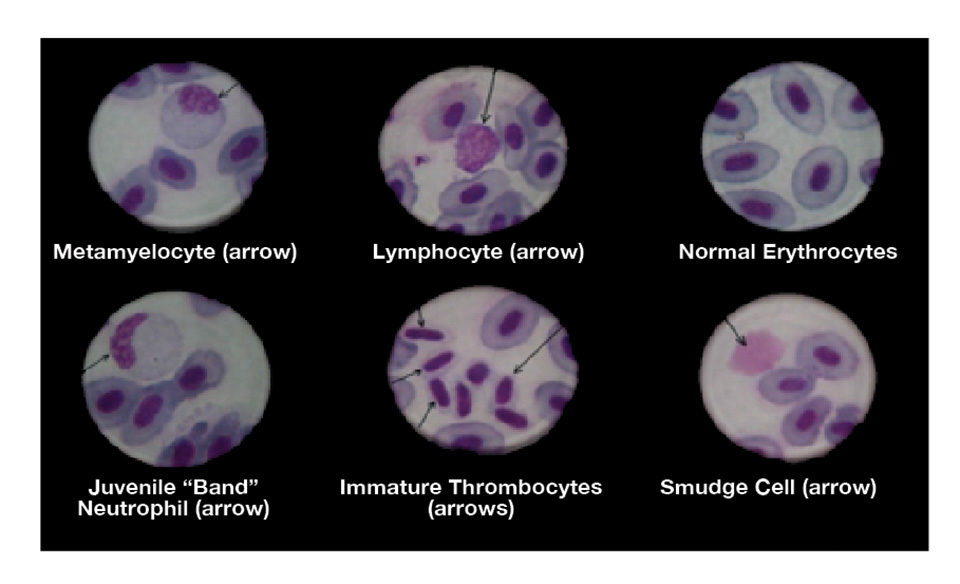
Hematological examinations are of increasing interest and importance in freshwater and coastal marine fish-farming operations worldwide. The results of these studies provide valuable information on the health status of the fish and usually include determinations of the hemoglobin content, numbers of red and white blood cells and hematocrit levels.
These data can then be used to calculate the principle hematological indices, all of which are helpful in examining aspects of the biology, nutrition, pathology, physiology and reproduction of the farmed fish species concerned.
Blood smears
An integral and particularly significant part of any hematological evaluation of a fish is the preparation, microscopic examination and interpretation of stained blood smears. By studying smears, the morphological appearance of the various normal and abnormal cellular elements present in the blood can be identified, and their relative numbers can be calculated in terms of their percentage distribution.
To achieve the best results, it is of fundamental importance that blood smears are correctly prepared and stained prior to their microscopic examination. Whenever feasible and in accordance with the size or life stage of the fish, blood smears should be prepared from samples of blood freshly obtained with a heparinized syringe for routine hematological examination purposes. Such samples are usually taken from the caudal vein or heart. In the case of fingerlings or other very small fish, it is generally sufficient to sever the caudal peduncle and use the drop of blood that appears as the sample.
A single drop of blood is placed on the surface of a clean and grease-free microscope slide at a distance of 2 cm from one end. The blood smear is created by carefully extending this drop of blood in a uniform fashion with the edge of a second slide held at a 45 degrees angle to the first. Once prepared, the blood smear slide is dried by gently waving it in the air.
When the smear is dry, the slide is marked with a diamond pencil or similar – not a grease pencil or marker containing ink that dissolves in alcohol – with information on the source and code number of the corresponding fish specimen from which the sample was taken. In cases where a bacterial infection is suspected, a second blood smear should also be prepared for subsequent staining by the Gram technique. The second test can detect any Gram-positive or Gram-negative bacteria present in the blood or phagocytosed within the macrophages.
Giemsa staining
The routine staining method that gives the best and most consistent results is the Giemsa technique. The air-dried blood smear is fixed in absolute methanol for five minutes and dried again in the air. It is then introduced, together with other similarly pretreated blood slides, into a staining jar containing a freshly prepared mixture of one part commercial liquid Giemsa’s stain in nine parts of buffered distilled water with a pH of 6.4.
The staining solution is allowed to act for 10 minutes, following which each smear is removed, carefully washed with distilled water, allowed to dry in the air and then examined microscopically using magnifications from 10 to 100 times actual size. It is important to emphasize that the staining solution must always be freshly prepared.
The examination procedure must include a minimum of 30 different microscope fields along the length of the entire smear so the results are representative and significant for each individual blood smear. The person undertaking the examination must, obviously, have a suitable degree of familiarity with the identification of the various cell types that might be encountered in the blood of the corresponding fish species.
The Giemsa staining technique identifies the following:
- Erythrocytes. Mature erythrocytes have a pale blue to pinkish-blue cytoplasm and a nucleus that stains bluish-purple. The cytoplasm of the polychromatocytes may show discrete areas that are only partially stained.
- Granulocyte leucocytes. The small granules in the cytoplasm of the neutrophils stain pink to lilac in color. The larger ones in the basophils and the eosinophils stain bluish-purple (basophilic) or orange-red (eosinophilic).
- Lymphocytes and thrombocytes. The cytoplasm stains a pale sky blue, and the nucleus is bluish-purple.
- Monocytes/macrophages. The cytoplasm stains pale blue, and the nucleus stains blue to purplish. There are often variably sized vacuoles in the cytoplasm, which may contain ingested bacteria, in the case of the macrophages.
Practical benefits
The practical benefits derived from the careful examination of stained fish blood smears are many, and the results have an important bearing on the diagnosis of certain nutritional, pathological and toxicological problems.
Nutritional problems
Vitamin E deficiency is often characterized by microcytosis and an abundance of “smudge cells” produced as a result of increased erythrocyte fragility. There are often reductions in the numbers of polychromatocytes and thrombocytopaenia that later impede normal blood coagulation.
Thiamine (vitamin B1) deficiency is associated with a microcytic anemia in which many of the polychromatocytes are found to be mitotically dividing in the peripheral blood.
Vitamin B12/folate deficiency is characterized by a hypochromic and often macrocytic anemia with evidence of division or segmentation of the erythrocyte nuclei. Dietary folate deficiency is characterized by normochromic macrocytes and dividing erythrocytes whose nuclei display an hourglass appearance. Iron deficiency anemia is also characterized by the presence of hypochromia and macrocytosis.
Pathological problems
The presence of inclusion bodies within the cytoplasm of the erythrocytes suggests viral infections of the viral erythrocytic necrosis and erythrocytic inclusion body syndrome types. Some bacterial infections show macrophages containing ingested bacteria, such as those caused by certain streptococci, Francisella species, rickettsia-like organisms, aeromonads and vibrios.
The presence of extra- and intracellular parasites such as haemoflagellates, haemogregarines and microfilaria of certain nematodes is readily confirmed in fish by the examination of suitably stained blood smears.
Toxicological problems
Cases of aflatoxicosis are generally characterized by microcytic anemia, poikilocytosis and the presence of large numbers of immature erythrocytic elements in the peripheral blood. As reported earlier by the authors, these elements give rise to a characteristic type of anemia in which such immature erythrocytic elements predominate.
Editor’s Note: The authors have prepared illustrated bilingual (English/Spanish) CD-ROMs to facilitate the recognition of normal and abnormal blood cells in Atlantic salmon and tilapia. They are also currently preparing English and Spanish language versions of a practical manual on hematological techniques for farmed fish species.
(Editor’s Note: This article was originally published in the July/August 2009 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Gina Conroy, M.Sc., C.Biol., F.I.Biol.
Director, Pharma-Fish SRL
Apartado de Correo #406
Maracay 2101-A
Edo. Aragua, Venezuela[116,101,110,46,118,116,110,97,99,64,53,48,48,50,103,105,110,97]
-
D.A. Conroy, Ph.D., C.Biol., F.I.Biol.
Emeritus Professor of Fish Pathology
Faculty of Veterinary Sciences
Central University of Venezuela
Related Posts

Health & Welfare
Common salt a useful tool in aquaculture, part 2
The preventive use of common salt (sodium chloride) by commercial producers of freshwater fishes has many benefits, including helping with the routine prevention of losses due to diseases, stress and mishandling during transport, harvesting, grading, counting, weighing and induced spawning.

Health & Welfare
Ongoing Vietnam studies find vibrio with phage transmits EMS/AHPNS
In 2012, samples collected from 92 AHPNS-affected ponds in the Mekong Delta found a number of Vibrio isolates, with the majority V. parahaemolyticus.

Intelligence
Post-harvest quality of freshwater prawns, part 2
Freshwater shrimp can contain pathogenic bacteria that cause illness unless care is exercised by producers, retailers and consumers. Many of the human pathogens can survive frozen storage, but are killed or inactivated by thermal processes.

Aquafeeds
Surprising results in rabbitfish diet evaluation
Soybean meal is not a viable option for partial or full fishmeal replacement in a diet for juvenile marbled rabbitfish. Results from a study in Beirut are somewhat surprising, as the herbivorous species is theoretically capable of performing well without animal-sourced protein in feed.


