Majority of gastroenteritis cases traced to noroviruses

As reported in previous articles in this series, noroviruses are the major cause of outbreaks of acute gastroenteritis and have been reported in hospitals, cruise ships, military and holiday camps, nursing homes, nurseries, hotels and restaurants. Epidemiological studies have estimated that noroviruses are responsible for 60 percent to 80 percent of all foodborne outbreaks of gastroenteritis worldwide. Consumption of shellfish is one of three main transmission routes of norovirus infection.
This article discusses the viruses’ presence and significance in seawater and shellfish. It is important to note that some molluscan shellfish in the study reporting were obtained from prohibited areas, areas known as receiving untreated sewage or from growing areas classified as B and C by the European Union Commission. Therefore, some of the studies may not reflect the potential for human illnesses due to harvest restrictions for direct human consumption.
It is also important to realize that shellfish viral outbreaks can be caused by multiple viruses. Following a 2006 flooding event close to a shellfish production lagoon in France, an outbreak of 205 cases of gastroenteritis was linked to oyster consumption. An analysis showed that of 12 stool samples collected from different individuals, eight were positive for multiple enteric viruses, and one stool sample contained seven enteric viruses. Shellfish were contaminated by as many as five different enteric viruses. For the first time in Europe, Aichi virus was detected in oyster samples.
Netherlands
In a study, shellfish from oyster farms in the Netherlands and shellfish imported from other countries (Germany, Ireland, Denmark, France and Britain) were examined for norovirus contamination. One of 21 oyster samples (4.8 percent) from Dutch farms was norovirus-positive, whereas norovirus was detected in one of eight oyster samples (12.5 percent) and five of 13 mussel samples (38.5 percent) collected directly after importation in the Netherlands.
Norovirus contaminations in the oysters were identified as genotype I.4 and in mussels as GI.4 and GIIb. The reason for the greater contamination in mussels was related to the possibility that the virus was recovered more efficiently from mussels than oysters.
Spain
An 18-month survey was conducted in 10 shellfish-harvesting areas in two regions in northwest Spain from January 2011 to June 2012. Each sample was composed of 10 mussels or 20 clams and cockles. The samples included wild and raft-cultured Mediterranean mussels, Mutilus galloprovincialis; Manila and carpet-shell clams, Venerupis philippinarum and Venus decussate; and cockles, Cerastoderma edule.
Overall, 55.4 percent of the samples were contaminated by at least one of the studied viruses – norovirus and hepatitis A – while 11.3 percent of the samples were contaminated by two or three viruses. At 32.1 percent, norovirus GI was the most prevalent virus, followed by norovirus GII at 25.6 percent and hepatitis at 10.1 percent. The results of the study are shown in Table 1.
Cultured mussels had the highest number of positive samples, followed by cockles and wild mussels. Viral contamination levels for most of the positive samples ranged from 102 to 103 RNA copies/g of digestive tissue. The presence of viral contamination was statistically higher in the warm months from April to September than in the colder months from October to March.
Italy
Samples of Mytilus galloprovincialis mussels, Tapes philippinarum clams and Crassostrea gigas oysters were collected from two harvesting areas in the delta area of the Po River in Italy. A total of 70 samples (35 for each area) were collected each month for one year for the determination of norovirus (NoV) GI and GII. Norovirus contamination was found in 51.4 percent of the samples, of which 2.9 percent contained only NoV GI and 14.3 percent contained only GII, while 34.3 percent of the samples contained both genotypes. About 90.0 percent of the positive results were obtained in the period between November 2008 and April 2009. There was no statistical difference between the frequencies of positive samples from the two harvesting areas.

At 60.9 percent, mussels showed the highest frequency of positive results, testing positive in 14 of the 15 samplings. The shellfish were similarly involved in the virus contamination, despite the different cultivation procedures – clams on the lagoon sand, mussels on ropes attached to stocks and oysters in floating cages.
In further study, from July 2007 to April 2010, 163 shellfish samples of shellfish were collected in the Campania region of southern Italy from harvesting areas, authorized and non-authorized retailers, and a restaurant following an outbreak of gastroenteritis. One hundred, seventeen Mutilus galloprovincialis mussels were obtained from 22 harvesting areas. Thirty-five mussel samples were obtained from retail stores, and 11 other shellfish species were obtained from other sources, including a restaurant that had a previous outbreak.
A total of 94 shellfish samples (57.7 percent) were positive for the presence of norovirus, and GII was the most frequently identified genogroup. Norovirus contamination was found in 74 percent of the shellfish purchased from retailers, and more than half of the samples from non-registered street vendors who sold unpacked shellfish were contaminated with norovirus.
However, in 46.7 percent of the samples of shellfish collected from harvesting areas and 55.9 percent of shellfish collected from retailers, norovirus from the GI and GII genogroups was found, indicating a high presence of norovirus GI in the environment. This finding is not consistent with data from human infections, which indicate an apparent worldwide dominance of norovirus GII, and could be related to a greater resistance to breakdown during water treatment of norovirus GI strains.
In 2008-2009 and 2011-2012, samples from 185 Mytilus galloprovincialis mussels, 66 Tapes philippinarum and Ruditapes decussates clams, 23 Crassostrea gigas oysters and 62 other Donax and Solen species were collected in harvesting areas of class A and B of four Italian regions and analyzed for norovirus GI and GII. Norovirus showed a high prevalence of 51.5 percent with a large variability according to the group considered – 47.8 percent in Crassostrea in Veneto, 79.7 percent for Mytilus in Campania and 84.6 percent for Tapes in Sardinia.
Norovirus contamination affected class A and class B production areas to a different extent, with a statistically significant difference in both contamination prevalence (22.1 percent versus 66.3 percent) and quantity (average contamination level of 3.1 x 102 versus 1.9 x 103 copies/g). The Mytilus, Tapes/Ruditapes and Crassostrea species analyzed from class B harvesting areas showed norovirus prevalence of 70.3, 66.0 and 47.8 percent, respectively. The other two bivalve species in the study, Donax and Solen, showed a relevant norovirus presence of 40.0 percent and 34.4 percent of samples, respectively.
British Isles
In research in the British Isles’ Pacific oysters, Crassostrea gigas, from commercial beds in two geographically distinct production areas were analyzed for the presence of noroviruses. Both harvesting areas were in estuarine locations with urban populations greater than 100,000 in the river catchment area and were potentially impacted by identifiable municipal sewer discharges serving those populations. A total of 237 samples were collected.
From production A, 145 samples were collected from January 2004 to July 2006, while from production area B, 92 samples were collected from January 2006 to July 2006, with a break from May to June 2005.
For production area A, 69 of 145 samples were negative for both norovirus GI and GII genotypes. Of the positive samples, 42 were positive for both genogroups, 18 were positive for GI only, and 16 were positive for GII only. For production area B, 29 of 92 samples were negative. Of the positive samples, 47 were positive for both genogroups.
Results in both areas showed strong seasonality, with peak monthly average norovirus contamination recorded between December and February and lowest levels generally recorded between June and August. Norovirus contamination was 17 times higher in oyster samples obtained from October to March than during the remainder of the year.
Authors
-

George J. Flick, Jr., Ph.D.
University Distinguished Professor
Food Science and
Technology Department
Center for Applied Health Sciences
Duck Pond Drive
Virginia Tech (0418)
Blacksburg, Virginia 24061 USA
[117,100,101,46,116,118,64,103,107,99,105,108,102]
-
David D. Kuhn, Ph.D.
Assistant Professor
[117,100,101,46,116,118,64,110,104,117,107,101,118,97,100]
Tagged With
Related Posts

Health & Welfare
Aquaculture viruses: An Atlantic salmon case study
Viruses often are the most potentially damaging pathogens in nature, affecting both wild stocks and farmed animals. Due to an Infectious Haematopoietic Necrosis (IHN) viral outbreak that occurred in Atlantic salmon in British Columbia, Canada some years ago, a vaccine for IHN was developed against this serious threat.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Born in Hawaii, SPF broodstock shrimp industry faces globalization
The next step for shrimp breeding will be developing animals that aren’t just disease-free, but increasingly resistant to multiple pathogens. The industry is globalizing, with suppliers setting up shop overseas. But its birthplace will always be Hawaii.
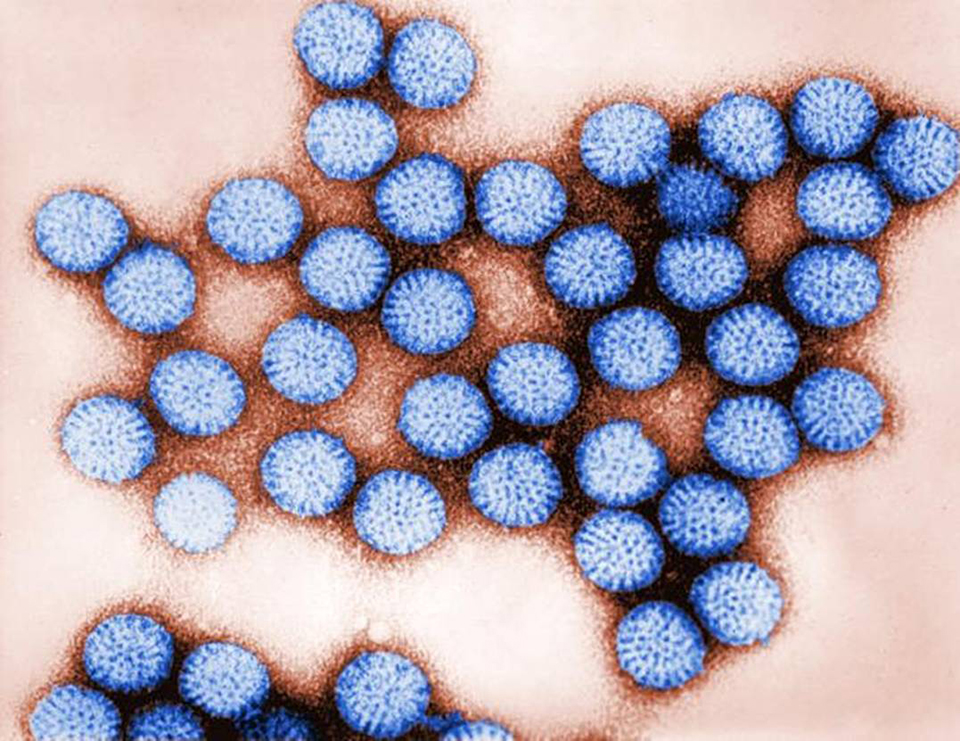
Intelligence
Human enteric viruses in shellfish, part 1
Commercially harvested shellfish have been reported to cause gastro-enteritis when humans consume virus-contaminated products. Rotaviruses are one of the main types of viruses able to survive and persist in the aquatic environment.


