High-quality, highly nutritious and biosecure live feeds are critical

As with all commercially produced marine finfish species, a predictable and sustainable supply of high-quality juveniles is critical for the expansion of commercial production of cobia (Rachycentron canadum). While great strides have been made over the last two decades, resulting in global cobia production of approximately 30,000 metric tons (MT) by 2008, fingerling production had already been identified as a major bottleneck towards significant increases in production.
Since the 1990s, both academic and industrial entities have struggled with improving the survival rates of cobia fingerlings. In the early 1990s, Asia adapted extensive production techniques used with other marine species and succeeded in consistent, low-density production of cobia fingerlings. This initial success enabled further development and expansion of cobia growout production techniques in Southeast Asia.
Around the turn of the century, significant interest in cobia culture expanded to the Americas. Specifically in North America, interest lay in more intensive and biosecure controlled production of specific pathogen-free fingerlings.
Cobia coalition
Beginning in 2001, Virginia Tech integrated cobia into its marine finfish production program, focused at the Virginia Seafood Agricultural Research and Extension Center (VSAREC) in Hampton, Va., USA. In 2005, Virginia Tech mariculture programming integrated with the International Initiative for Sustainable and Biosecure Aquafarming (IISBA), which was formed after an international cobia summit held in the European Union.
Since 2005, this coalition has focused primarily on the fingerling production bottleneck for cobia, which was defined by an inability to consistently produce an average survival rate of 25 percent or greater, and a final production of 5 fingerlings/L. While every year since 2005 resulted in improved production techniques, protocols, systems and biosecurity measures, this production bottleneck persisted until 2009.
In 2009 and again in 2010 with even better results, this coalition validated intensive cobia larviculture production systems and protocols that finally broke the fingerling production bottleneck. With these refinements, survival rates of over 35 percent from stocking sac fry through weaning, with an average production of 5.5 fingerlings/L in 25 days were achieved. In addition, these were high-health juveniles produced in biosecure systems, a requirement for sustainable industrial expansion of intensive cobia growout.
These new production protocols and procedures represent the combined inputs and efforts from numerous individuals, academic institutions and industry partners, all aligned under the IISBA umbrella.
Live feeds production
Critical to the larviculture production procedures is the use of high-quality, highly nutritious and biosecure live feeds. To accomplish this, strict protocols have been developed regarding the culture, enrichment, harvest and storage of roti-fers and artemia.
Rotifers are produced in an automated generator comprised of two 500-L culture tanks, a fluidized-bed biofilter and a protein skimmer. The system is operated with a single 0.1-h.p. magnetic drive pump. Flow rates through the culture tanks range 0.5-1.0 L/minute.
This simplified production system can be operated on a continuous or batch basis, as dictated by the hatchery schedule. The rotifers are cultured on a commercial algae product from a dilution injected into the culture tanks via a peristaltic pump from an adjacent small refrigerator every 120 minutes. Rotifers are then enriched using commercial products in a separate tank prior to being fed to larvae.
Artemia, while not close in size, movement or nutritional composition to natural prey items for cobia, are presently recognized as the most reliable, predictable and available transitional live feed used in larviculture between rotifers and dry diets. Given the limitations of this food item, it is critical to make sure artemia are of the highest health and nutritional value when fed to the cobia.
Toward this end, Virginia Tech production protocols incorporate decapsulation, hatching, membrane flotation, Artemia enrichment with a commercial product and subsequent cold-storage techniques. It is worth noting that while not presently incorporated into the live feeds production protocols, recent results from replicated cobia larviculture research trials indicated that utilizing a commercially available probiotic additive resulted in improved salinity stress index, immunocompetence and physiological development.
Larviculture production
Fertilized eggs are incubated in a 200-L conical tank with 28 degrees-C recirculated water from the larviculture production system. After hatching, the sac fry are maintained in this incubator for another 24 hours for further development. Two days after hatching, the sac fry are stocked at 15/L into the larviculture production units, where they stay for the remainder of the production run.
Beginning on day 3, a commercial algal paste is added to the culture water every six hours throughout the rotifer-feeding stage until transition to the second prey item, artemia, is complete. Previous work conducted at Virginia Tech demonstrated the efficacy of concentrated algal paste as a replacement for live algae.
The production tanks are operated in recirculation mode, allowing near-daily adjustments of the flow rates based upon feed type, feed quantity and the developmental stage of the larvae. Critical to this protocol is the utilization of a pulse feeding approach.
Following this method, live feeds are added to the production tanks multiple times per day – in this case, every six hours. Water-exchange rates are balanced with prey densities to insure live feeds are either consumed or flushed from the tank within four hours of feeding. This provides a brief time period in which the larvae have no food sources to ingest, allowing more complete digestion between feedings.
During the transitions between live feeds and from live feeds to dry diets, a process of co-feeding is employed for a five-day period. The three-day weaning process, which typically begins 20 days post-hatch and involves a stepped decrease in the amount of enriched artemia introduced to larvae, is coupled with an increase in commercial dry diet additions.
Due to the frequency of algae, live feed and dry feed additions during the critical co-feeding and weaning procedures, the use of automated feeders has been implemented to reduce labor. Programmable peristaltic pumps precisely dispense algae, rotifers and artemia from cold storage to culture tanks, while an automatic microdiet dispenser system is used for dry diet additions.
Larviculture system, operations
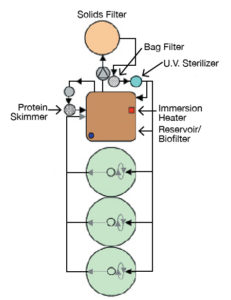
The larval production systems (Figure 1) at VSAREC are comprised of 3 tanks/system, with volumes of 300 L for research and 1,000 L for production. In each system, larviculture tanks are connected via gravity to a reservoir that doubles as a fluidized biological filter. From this fluidized reservoir, water is pumped via a 0.3-h.p. pump to an upflow bead filter for solids removal.
From the bead filter, water flows through a canister filter with bags sized for the feed items used. From the canister, the water flows through an 80-watt ultraviolet filter and then to a distribution manifold, which takes water to the larval tanks via individual flow meters or shunts it back to the reservoir. This configuration allows daily flow adjustments to the larval tanks without negative impacts on the efficiency of the water treatment system.
During production runs, the bead filters are backwashed once daily to remove solids and maintain optimal water quality parameters in a process that equates to about 10 percent daily exchange. Makeup water is surface water pumped from the Chesapeake Bay and filtered, chlorinated and dechlorinated prior to use.
A critical component of the larval production tanks is their cross-sectional width:depth ratios, which should be around 2.5:1. A ratio of 3:1 is preferred during the greenwater/rotifer stage. In addition, the hydrodynamics of the culture tanks need to be carefully addressed for maximum cobia production.
Through the strategic placement of airstones and influent water pipes, both horizontal and vertical circulation cells are developed throughout the water column that facilitate distribution of live feeds as well as larvae throughout the tank. As cobia larvae develop, these circulation cells are increased to minimize cannibalistic behavior and subsequent mortality.
Projected output
Ten MT of production tanks can produce approximately 55,000 fingerlings every 23 days. With a conservative growout cycle of 12 months and a post-larviculture survival rate of 80 percent, a final production weight from this recruitment would yield about 167 mt of cobia. Given the amenability of cobia fingerlings to traditional cold-banking techniques for periods of up to three months with no subsequent lack of performance, a cobia facility with 1,000-MT annual production would need to conduct four larval production cycles in a year, with a hatchery capacity of 15 MT.
As research continues aggressively for this species, future improvements in system and fish performance efficiencies are anticipated. The present protocol improvements under study by the Virginia Tech/IISBA coalition are focused on concurrent reduction of live feed requirements and a shortening of the live feed periods during larviculture.
(Editor’s Note: This article was originally published in the May/June 2011 print edition of the Global Aquaculture Advocate.)
Authors
-
Michael H. Schwarz, Ph.D.
Virginia Tech
102 S. King Street
Hampton, Virginia 23669 USA[117,100,101,46,116,118,64,122,114,97,119,104,99,115,109]
-
Brendan C. Delbos
Virginia Tech
102 S. King Street
Hampton, Virginia 23669 USA
Related Posts

Health & Welfare
Cobia culture in recirculating systems
A unique pilot project supported by northern Chile’s biggest power-generating company and the Undersecretary of Fisheries and Aquaculture is raising cobia in a recirculating aquaculture system in the desert.

Innovation & Investment
Artemia, the ‘magic powder’ fueling a multi-billion-dollar industry
Artemia, microscopic brine shrimp used as feed in hatcheries, are the unsung heroes of aquaculture. Experts say artemia is still inspiring innovation more than 50 years after initial commercialization. These creatures are much more than Sea-Monkeys.

Intelligence
Bahamas venture focuses on grouper, other high-value marine fish
A new venture under development in the Bahamas will capitalize on Tropic Seafood’s established logistics and infrastructure to diversify its operations from processing and selling wild fisheries products to include the culture of grouper and other marine fish.

Health & Welfare
Tropical, subtropical marine fish hatchery technology needs improvement
Hatchery technology for most commercially important species of cultured marine fishes is essentially unchanged for the past several decades.


