Some antimicrobial compounds appear to disrupt bacterial communication that activates genes associated with release of toxins

As shrimp aquaculture expands its global reach, the industry continues to be plagued by various diseases caused by viruses and bacteria. Attempts to treat shrimp infections with chemical therapeutics and antibiotics at commercial farms frequently fail, and their prophylactic use in intensive systems can cause the emergence of resistant microbial strains, environmental pollution, accumulation in tissues and immunosuppression in shrimp.
Vaccination is an effective prophylactic method against microbial infections in fish, but not in shellfish. Additionally, there are methodological issues, such as the cost involved and the induced stress for shellfish. Hence, there is a need for disease preventative measures to promote a more sustainable shrimp culture.
One of the more promising methods for controlling diseases is to strengthen non-specific defense mechanisms in shellfish. The level of disease resistance can be enhanced by the use of immunostimulants introduced through enriched feeds, thus obviating the use of antibiotics and chemotherapeutic agents.
The authors recently performed a study to evaluate the onset and duration of immunity to white spot syndrome virus (WSSV) and a virulent strain of Vibrio parahaemolyticus capable of inducing early mortality syndrome in shrimp treated with a newly developed mineral extract used as a feed additive at different concentrations in pelletized formulations.
Pathogens
A proven-virulent V. parahaemolyticus isolate was provided by Dr. Bruno Gómez of Centro de Investigación en Alimentación y Desarrollo (Center for Food Research and Development) in Sonora, Mexico. The WSSV isolate, which originated from infected L. vannamei at a commercial farm in Sinaloa, Mexico, was serially diluted to prepare solutions containing various target copy numbers determined by polymerase chain reaction (PCR). Shrimp were injected intramuscularly in the fourth or fifth abdominal segment with 20 μL of different dilutions of the standard tissue homogenate in salt water. Dead shrimp were tested for the presence of WSSV by PCR.
Previous studies showed that a WSSV dilution containing 1 x 106 copies was appropriate for use in subsequent challenge trials. This study found that a virus diluted at 3 percent could kill 50 percent of the shrimp in 48 hours and 100 percent in 96 hours. On the other hand, a 1 percent WSSV suspension could kill 80 percent of the shrimp in 120 hours.
Initial culture
Twelve to 15 days after their hatching, shrimp postlarvae were obtained from a commercial hatchery in La Paz, Baja California Sur, Mexico, and stocked into 1,000-m2 lined ponds at 60/m2, where they were reared using standardized photoautotrophic techniques and fed a 35 percent-protein commercial pelletized diet three times daily.
At a weight of 3 ± 1 g, the shrimp were transferred indoors to 1,000-L plastic tanks filled with filtered, ultraviolet-treated and aerated 37-ppt seawater at temperatures of 27 to 30 degrees C. Partial water exchanges were made once a week to remove uneaten food and feces, and maintain water quality.
When shrimp reached 5 ± 1 g, they were fed the pelletized diet ad libitum in replicated experimental units. However, the diet was reformulated so that each gram of feed contained 1, 5 or 10 mg of the mineral extract feed additive. After 15 days, shrimp were transferred to a biosecure laboratory for the challenge trials.
Challenge study
An experimental system consisting of 48, 30-L plastic tanks was used. Each tank had an air stone for aeration of the sterilized seawater, conditioned to 27 degrees C and a pH of 8. Twenty shrimp were introduced to each tank and left to adapt to the system for two days before initiating the trial.
The shrimp were challenged in triplicate by injection with a known concentration of WSSV or a virulent V. parahaemolyticus strain. Positive control groups of shrimp that were not previously exposed to diets containing the mineral extract were also injected with the pathogens.
A negative control group of shrimp not fed the experimental diets was not challenged with pathogens, but was injected with a saline solution. After injection, the treated shrimp continued receiving the same diet ad libitum, while the controls received the pelletized commercial feed.
Results
WSSV
Test results showed statistical differences (P < 0.05) in the survival of the shrimp challenged with a 3 percent WSSV suspension (Fig. 1). After 48 hours, shrimp from the positive control had 80 percent survival, while shrimp given the feed with 5 mg additive/g had 95 percent survival. After 96 hours, only 10 percent of the positive control shrimp were alive. There was a marginal improvement for shrimp fed the diets with 1 and 5 mg mineral extract/g, with survivals over 30 and 20 percent, respectively.
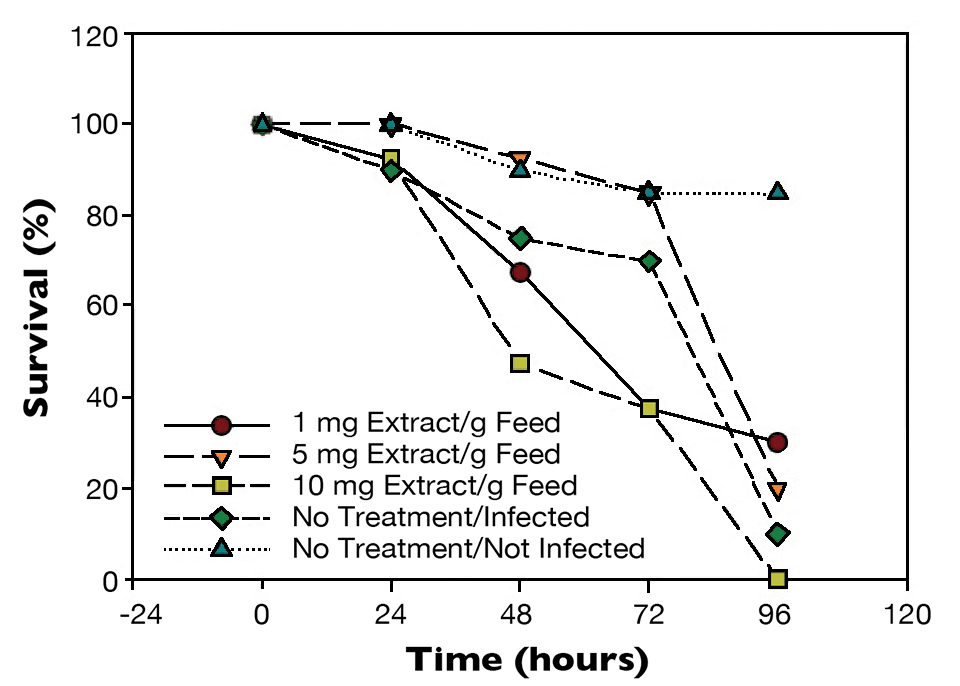
Similarly, when shrimp were challenged with a 1 percent WSSV suspension (Fig. 2), there was a statistical difference (P < 0.05) in survival after 72 hours between shrimp from the positive control, which had 80 percent survival, and shrimp fed the 5 mg/g feed, which had 95 percent survival. After 120 hours, there were 20 percent survival for the non-treated WSSV-injected shrimp and 90 percent survival for shrimp given the diet with the higher level of mineral extract.
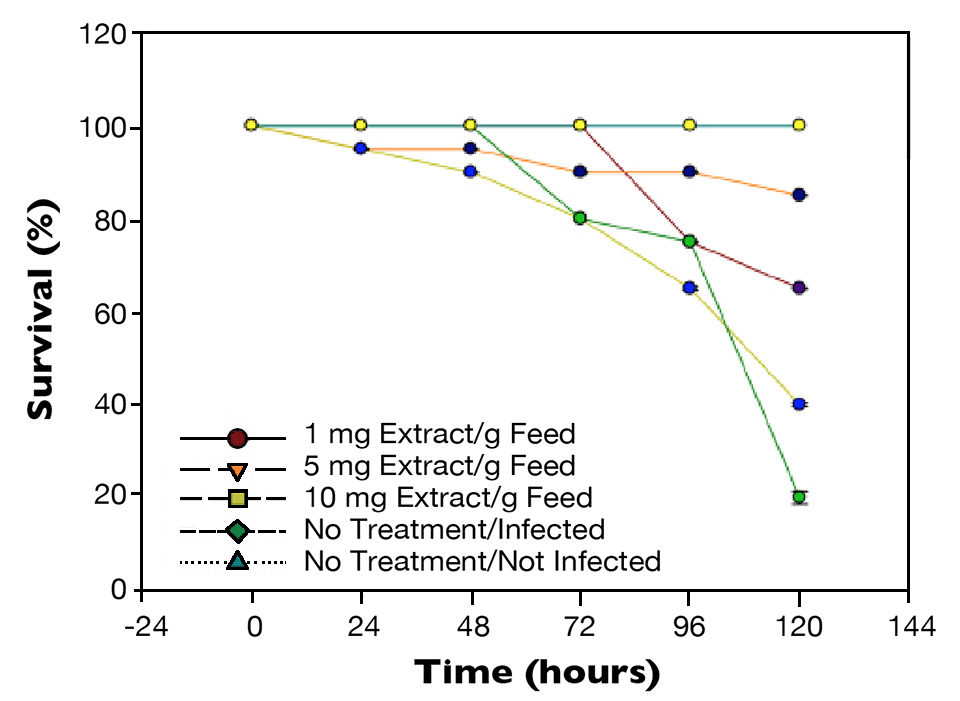
Vibrio parahaemolyticus
In shrimp challenged with a virulent V. parahaemolyticus strain (Fig. 3), survivals were statistically different (P < 0.01) after 24 hours. The positive control had 40 percent survival, and shrimp given the treated feeds had close to 100 percent survival.
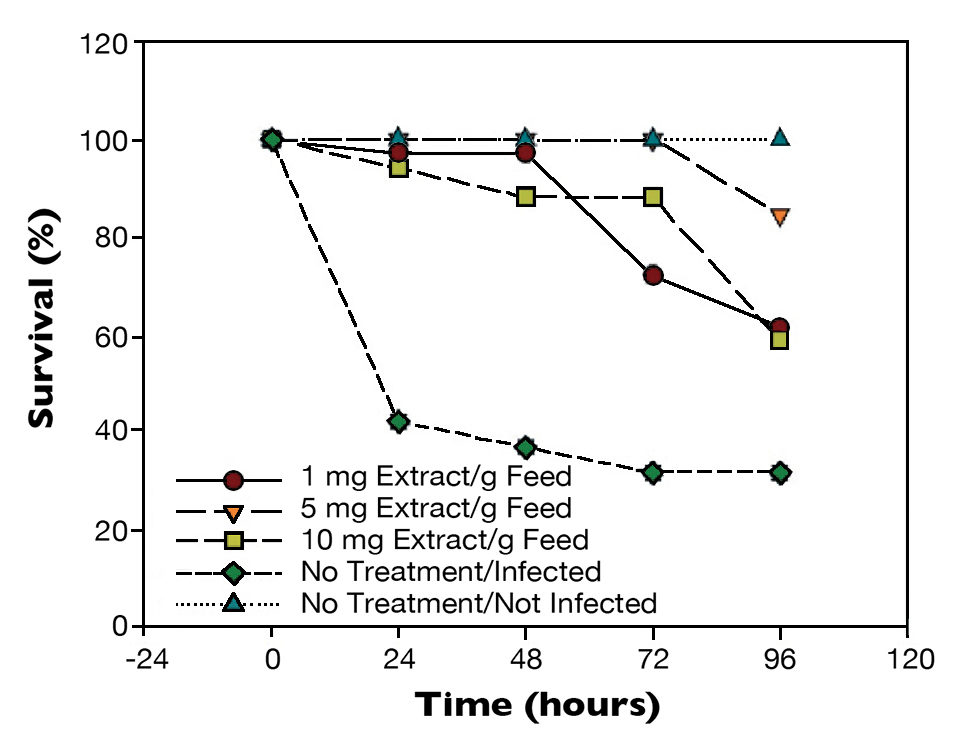
Results at 96 hours post-challenge showed 30 percent survival for the infected control. Shrimp fed 1 or 10 mg extract/g feed had 60 percent survival (P < 0.05), while shrimp fed the 5 mg/g feed had 90 percent survival (P < 0.01).
Perspectives
The use of functional feeds that contain antimicrobial compounds is increasingly considered fundamental in strategies to prevent infectious diseases in shrimp, such as early mortality syndrome and white spot syndrome. Further, some antimicrobial compounds, such as the one tested in this trial, appear to disrupt the bacterial communication that activates certain genes associated with the release of toxins. This quorum quenching represents a significant alternative to the use of antibiotics.
These results showed that the incorporation of a mineral extract at a 5 mg/g concentration in pelletized diets was effective in reducing the impacts of WSSV infection when following standardized injection protocols. The compound is stable, simple to administer and relatively inexpensive. Its inclusion in commercial diets provides an efficient mechanism for microbial activity control in the shrimp gut that may contribute to the solution for the mass mortalities associated with V. parahemolyticus and WSSV in commercial shrimp farms.
(Editor’s Note: This article was originally published in the January/February 2015 print edition of the Global Aquaculture Advocate.)
Authors
-
Humberto Villarreal-Colmenares, Ph.D.
BIOHELIS Innovation and Technology Park
Centro de Investigaciones Biológicas del Noroeste, S.C.
Instituto Politécnico Nacional #195
Col. Playa Palo de Santa Rita
La Paz, Baja California Sur, México
23090[120,109,46,114,111,110,98,105,99,64,52,48,111,116,114,101,98,109,117,104]
-
Felipe Ascencio-Valle, Ph.D.
Centro de Investigaciones Biológicas del Noroeste, S.C.
Instituto Politécnico Nacional #195
Col. Playa Palo de Santa Rita
La Paz, Baja California Sur, México
23090
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Genetic variation for resistance to WSS, AHPND in Pacific white shrimp
Selection for disease resistance has been used in breeding farm animals and can be a viable option to deal with white spot syndrome and acute hepatopancreatic necrosis disease in commercial shrimp culture. In trials, heritability for AHPND resistance was low, while that for WSS was moderate.

Health & Welfare
Potential applications of bacteriophages for AHPND control
Study demonstrates that isolated phages tested are effective in controlling AHPND infection in farmed penaeid shrimp and inhibiting bacterial growth.


