Hatcheries should aerate water before bringing it into facilities

Approximately 1.8 billion channel catfish fry are hatched each year in the United States, many of them in the state of Mississippi. Both the hatch rates and survival through sac fry stage of these catfish fry can be highly variable. The authors recently conducted studies to determine the effects of dissolved-oxygen concentration on the hatching and survival of channel catfish to swim-up stage.
Hatchery operations
With hatch rates averaging near 80 percent, the designs of current U.S. catfish hatcheries are not much different from those used when the industry first began. With proper management, up to half the females in commercial brood ponds spawn each year.
Catfish brooders at least 3 years old are moved into newly prepared ponds prior to the spawning season. Spawning cans are placed in the ponds and checked every few days during April through June in the southern United States. Spawns are removed from the cans and taken to the hatchery for incubation.
Incubation and hatching of the adhesive eggs is straightforward. Egg masses are suspended in hatchery troughs in baskets made with mesh plastic or metal screen. Water is flushed through the troughs, and the eggs are agitated by paddles that rotate on shafts suspended over the troughs. This agitation is intended to mimic the care normally provided by the male catfish, which guard, aerate, and agitate the spawn in nature.
When the eggs hatch, individual sac fry fall through the basket mesh and sink to the bottom of the troughs. They are siphoned up, measured volumetrically, and placed in rearing troughs until they reach swim-up stage and begin to feed. After feeding for a few days, they are stocked into 2- to 4-hectare ponds, where they remain until they are sold as fingerlings.
Oxygen treatment study
In the first study, several channel catfish spawns were split into two equal portions and incubated in tanks aerated with either air (low-oxygen treatment) or liquid oxygen (high-oxygen treatment). Dissolved-oxygen (D.O.) levels averaged 7.4 milligram per liter (94 percent saturation) and 18.4 milligram per liter (233 percent) through hatching in the low- and high-oxygen treatments, respectively.
Eggs hatched six hours earlier with low-oxygen incubation, but reached swim-up stage 31 hours later. Survival to swim-up stage in the low-oxygen treatment was reduced by 18.5 percent. Since most publications on catfish hatchery water quality recommend a dissolved-oxygen concentration of 5-6 milligram per liter (62-75 percent saturation), these results were difficult to explain until a second study was conducted.
Metabolism study
In the second study, routine metabolism (oxygen consumption) was determined on eggs, sac fry, and swim-up fry using closed respirometry. Metabolism reached 200 mg oxygen per kilogram-hour (wet weight basis) on the last day of egg incubation (Fig. 1), but sac and swim-up fry metabolism continued to increase to a maximum of 1,400 mg oxygen per kilogram-hour by about the fifth day after swim-up. At this point, metabolism began to decrease because an increasing portion of the fry was comprised of non- or low-metabolizing tissues, and growth as a percentage of weight decreased.
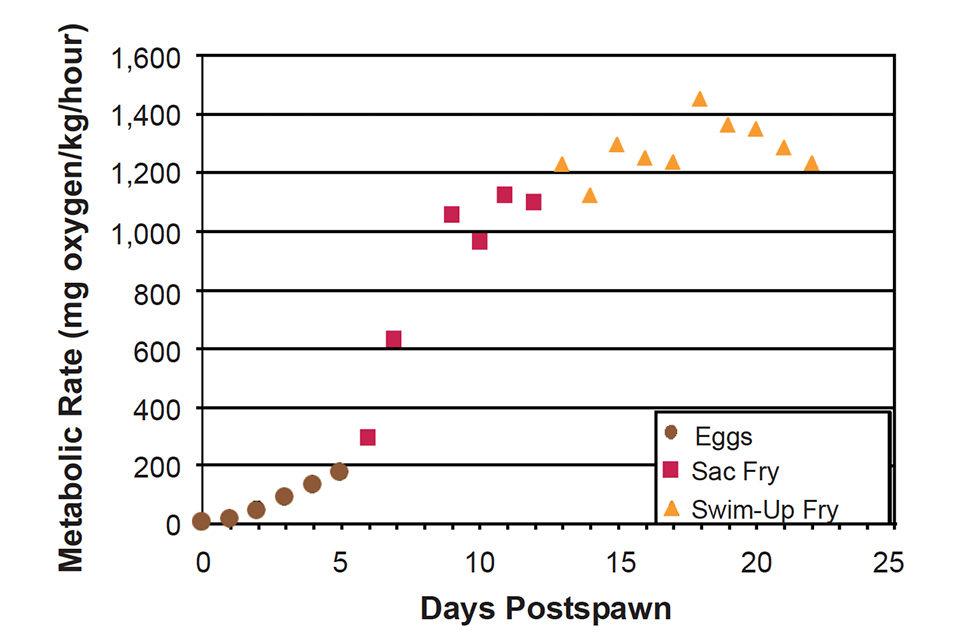
At higher oxygen concentrations, fish metabolism is generally independent of oxygen concentration. However, as oxygen saturation decreases, a point is reached where metabolism is affected. As the oxygen concentration decreases further, so does metabolism. This point is called the critical oxygen level or incipient limiting oxygen level, which was also determined during routine metabolism measurements on eggs and fry.
Results
The critical oxygen levels increased during egg incubation and peaked during the last day of egg incubation at 96 percent saturation (Fig. 2). Immediately after hatching, when the diffusion barriers of the egg shell and the water within it were lost, the critical oxygen levels for sac fry decreased to less than 50 percent saturation.
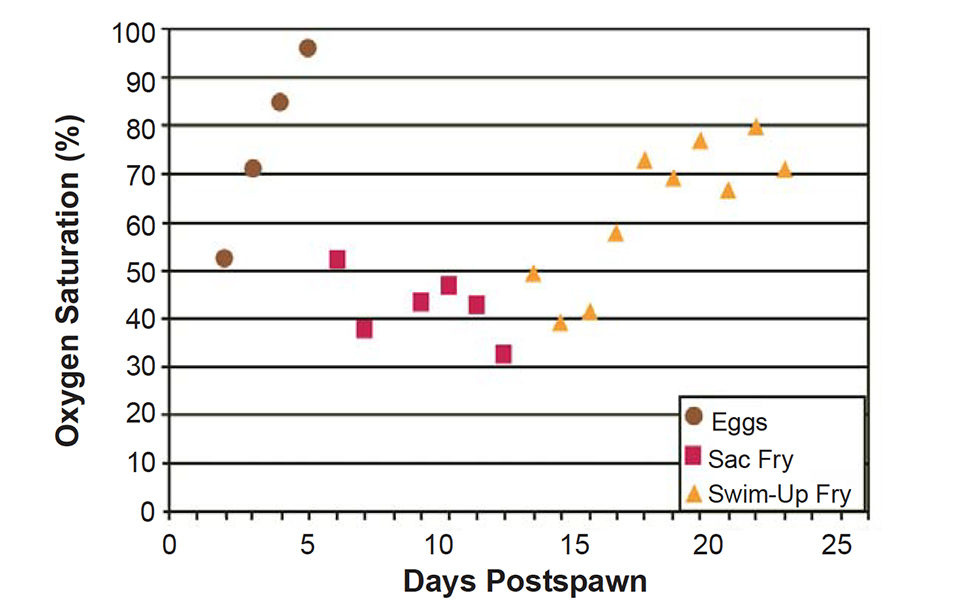
Critical oxygen levels increased over time as sac and swim-up fry metabolism rapidly increased, but plateaued at 70-80 percent saturation, much lower than that required for eggs near hatching. So although swim-up fry have a metabolic rate seven times greater than eggs, they require a much lower environmental dissolved-oxygen concentration to maintain it.
Premature hatching observed in the low-oxygen treatment in the first study was likely precipitated on the last day of incubation, when the 94 percent oxygen concentration to which the eggs were exposed was less than the 96 percent level required to maintain maximum egg metabolism. While this premature hatch apparently resulted in 18.5 percent poorer survival to swim-up stage, this mechanism would be adaptive in nature, where hatching early, even with a reduced survival, would be preferable to the potential total loss of the egg mass from low oxygen.
As further evidence, the authors observed spontaneous hatching of egg samples in the respirometer on the last day of incubation, when the dissolved-oxygen concentration decreased. Catfish hatchery operators have also noted this phenomenon when aeration/agitation of eggs in the hatchery ceased due to an electrical outage.
It is unlikely hatchery operators would detect premature hatching of some spawns. Eggs in a spawn normally hatch over a period of several hours. In addition, spawns differing one to three days in age may be put in the same hatching trough, since spawning cans are rarely checked daily. Thus, if some eggs hatched six to 12 hours prematurely, it would likely go unnoticed, even if those fry had a 10-20 percent poorer survival to swim-up. Such losses would be written off as normal variation.
Field survey
While the laboratory studies took place, a field survey of 26 commercial catfish hatcheries in the Mississippi River delta was also conducted. The hatcheries sampled represented approximately 40 percent of the annual hatching capacity of the U.S. catfish industry.
Oxygen saturation in the hatching troughs sampled ranged 45.2-100.2 percent. Nearly half the hatcheries (12 of 26) maintained dissolved oxygen at less than 90 percent saturation, while only seven held D.O. at over 96 percent saturation.
Since the results of this study were presented to various industry groups, several hatchery managers have commented on the highly variable but unexplained mortality seen during the sac fry stage. Some have also noted that this problem is more severe during peak spawning periods, when the hatchery troughs are more crowded and more likely to have lower dissolved oxygen as the eggs near hatching. The likely explanation is that premature hatching may be precipitated by D.O. concentrations of 75-90 percent saturation, and fry hatching even a few hours early will have poorer survival to swim-up.
Monitor oxygen saturation
The authors recommend that hatcheries aerate water before bringing it into the facilities by running it through a packed column. This increases the initial oxygen saturation and helps remove any undesirable gases sometimes found in well water. Many hatcheries also have blower systems that further aerate water with diffusers placed in the troughs.
Managers should routinely monitor dissolved-oxygen levels throughout hatcheries and correct them if the oxygen levels fall much below saturation as hatching nears. The addition of a second line supplying pure oxygen to even one or two diffusers per trough could increase the D.O. to slightly above saturation. The cost of oxygen would be insignificant, since this would be used only during the last day of egg incubation.
The authors believe it is possible the production of swim-up fry across the U.S. industry could be increased 200-400 million per year by maintaining higher oxygen saturation in hatchery troughs during the last day of incubation.
(Editor’s Note: This article was originally published in the June 2006 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Les Torrans, Ph.D.
USDA ARS Catfish Genetics Research Unit
Thad Cochran National Warmwater Aquaculture Center
P.O. Box 38
Stoneville, Mississippi 38776 USA[118,111,103,46,97,100,115,117,46,115,114,97,64,115,110,97,114,114,111,116,108]
-
Jim Steeby, Ph.D.
Mississippi State University Extension Service
Belzoni, Mississippi, USA
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.

Health & Welfare
Antigens provide immunity against ich in channel catfish trials
Vaccination against the Ich parasite is an alternative to chemical treatment. Fish develop a humoral immune response to trophont antigens, with the degree of protection related to the immunizing doses of trophonts used.

Innovation & Investment
Assessing coloration in channel catfish fillets
Because consumers look at color to gauge quality of catfish fillets, the authors developed a digital photography measurement method to assess yellowness.


