Mucus, epidermis are first lines of defense against invasive microorganisms

In aquaculture systems, fish are commonly infected by multiple pathogens. The Gram-negative bacterium Flavobacterium columnare and the parasite Ichthyophthirius multifiliis are two common pathogens of cultured tilapia. The optimum temperature ranges for the organisms overlap at 20 to 25 degrees C, which explains why their effects are often seen together.
F. columnare is the causative agent of columnaris disease. Columnaris is generally regarded as an external infection with clinical signs of skin lesions, fin erosion and gill necrosis. Many commercially important freshwater fish worldwide are susceptible to columnaris, which can result in high mortality.
I. multifiliis, commonly referred to as “Ich,” is a protozoan parasite in various freshwater fish worldwide. The parasite damages fish gills and skin, results in high fish mortality and leads to substantial economic losses for aquaculture. The life stages of Ich include an infective theront, a parasitic trophont and a reproductive tomont.
A study by the authors evaluated whether hybrid tilapia infected with I. multifiliis were more susceptible to F. columnare. Bacterial numbers in gill and kidney tissues were also compared between parasitized and non-parasitized fish.
Fish, parasite, bacterium
Sex-reversed hybrid tilapia were used as experimental fish because they are commonly cultured in intensive production. The tilapia fry were reared to experimental size – 9 cm in length and 12 g in weight – in indoor tanks at the Aquatic Animal Health Research Unit of the United States Department of Agriculture Agricultural Research Service in Auburn, Ala., USA.
The I. multifiliis strain used was isolated from an infected fish from a local pet shop and maintained by serial transmission on channel catfish. To culture theronts for the infection trial, heavily infected fish with maturing I. multifiliis were anesthetized and rinsed in tank water. The skin was gently scraped to dislodge the parasites. Isolated trophonts were placed in a tank with 20 L water and incubated at 22 to 24 degrees C.
Two isolates of F. columnare were used in this study: ALM-05-53 obtained from a channel catfish and TN-3-2012 isolated from a hybrid tilapia. The isolates were inoculated in modified Shieh broth and incubated aerobically on a shaker set at 28 degrees C for 24 hours. Following 24 hours of growth, the concentration of the bacterium was determined by bacterial plate count to define the number of colony-forming units (CFUs) per milliliter.
F. columnare infection trial
The fish were divided equally into four tanks with 100 fish/tank for parasite infection. I. multifiliis was added to each of two tanks at 30,000 theronts/fish. The tilapia were exposed to theronts for one hour with aeration. The fish in the other two tanks were not exposed to I. multifiliis theronts, but kept in water for one hour with aeration.
Five days after the theront exposure, 10 fish from each tank were inspected for parasite infection by wet mount from caudal fins under a microscope. All examined fish from the infected tanks showed light infections of I. multifiliis. The remaining fish were divided into 18 tanks with 20 fish/tank.
Treatments included a non-infected control, tanks infected by I. multifiliis alone, tanks infected by F. columnare ALM-05-53, tanks infected by I. multifiliis and exposed to F. columnare ALM-05-53, tanks infected by F. columnare TN-3-2012, and tanks infected by I. multifiliis and exposed to F. columnare TN-3-2012.
For challenge with F. columnare, fish were immersed in water in buckets with ALM-05-53 or TN-3-2012 at 4 x 107 CFU/mL for 15 minutes. Fish not exposed to the bacteria were kept in water with Shieh broth for the same duration.
F. columnare in fish tissues
Three days after the challenge, gill and kidney tissue from two fish randomly sampled from each tank were collected for F. columnare quantification. Each tissue sample of about 20 mg was macerated with a sterilized pestle in a microcentrifuge tube. DNA was extracted and purified.
Real-time polymerase chain reaction (qPCR) testing was used for the quantitation of F. columnare in infected fish. The qPCR was performed using two F. columnare-specific primers and a dual-labeled probe. Bacterial DNA in each milligram of tissue was determined as genome copies per milligram of tissue.
Results
The fish showed 2.1, 0 or 29.1 percent mortality when infected by I. multifiliis alone, with F. columnare TN-3-2012 alone or with F. columnare ALM-05-53 alone (Table 1). The parasitized fish showed 25.0 percent and 60.4 percent mortality after exposure to F. columnare TN-3-2012 and ALM-05-53, respectively.
Xu, Cumulative mortality, Table 1
| Theronts/fish | F. columnare Isolate | Number of Fish | Dead Fish | Mortality (percent) |
|---|---|---|---|---|
| 0 | No | 48 | 0 | 0 |
| 30,000 | No | 48 | 1 | 2.1 |
| 0 | ALM-05-53 | 48 | 14 | 29.1 |
| 30,000 | ALM-05-53 | 48 | 29 | 60.4 |
| 0 | TN-3-2012 | 48 | 0 | 0 |
| 30,000 | TN-3-2012 | 48 | 12 | 25.0 |
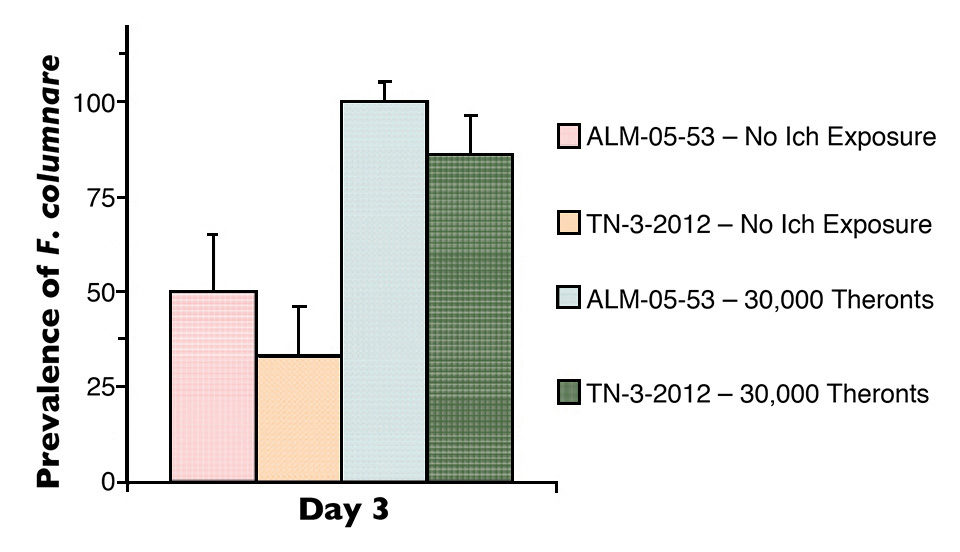
Mortalities were significantly higher in I. multifiliis-parasitized fish than the non-parasitized fish after exposure to F. columnare. F. columnare was isolated from 33 to 50 percent of non-parasitized tilapia and from 83 to 100 percent of parasitized tilapia three days after exposure to F. columnare (Figure 1).
The bacterial numbers increased significantly in the gill tissues of parasitized fish compared to those of non-parasitized fish after exposure to F. columnare. The bacterial number in gill tissue of parasitized fish (5,702 genome equivalents/mg) was 14-fold higher than the level found in non-parasitized fish (415 G.E./mg) three days following exposure to F. columnare ALM-05-53 (Table 2). The parasitized fish showed a bacterial load of 2,526 G.E./mg, which was 13-fold higher than the 197 G.E./mg load in non-parasitized fish after exposure to F. columnare TN-3-2012.
Similarly, the kidneys of parasitized fish showed significantly higher bacterial numbers than those of non-parasitized fish after exposure to F. columnare (Table 2). The bacterial number in kidney tissue of parasitized fish was 17-fold higher than that of kidney tissue from non-parasitized fish after exposure to F. columnare ALM-05-53.
Xu, Genome copy of F. columnare, Table 2
| Tissue | Theronts/Fish | F. columnare Isolate | GE/mg |
|---|---|---|---|
| Gill | 0 | ALM-05-53 | 415 |
| Gill | 0 | TN-3-2012 | 197 |
| Gill | 30,000 | ALM-05-53 | 5,703 |
| Gill | 30,000 | TN-3-2012 | 2,526 |
| Kidney | 0 | ALM-05-53 | 472 |
| Kidney | 0 | TN-3-2012 | 84 |
| Kidney | 30,000 | ALM-05-53 | 8,180 |
| Kidney | 30,000 | TN-3-2012 | 1,587 |
Perspectives
The mucus and epidermis of fish are the first lines of defense in protecting against invasive microorganisms. Fish mucus contains a variety of antimicrobial compounds, such as antibacterial peptides, lysozyme, proteases and antibodies that protect underlying epidermal cells from bacterial adhesion and colonization.
Parasite infection by I. multifiliis resulted in damage to the fish skin and gills. When the burrowing theronts move between epithelial cells to seek sites for adherence, they push epithelial cells apart and cause cell injury.
This study demonstrated that parasitic infection enhanced bacterial invasion, resulted in high numbers of bacteria in fish tissues and subsequently increased fish mortality. This work suggested that the prevention of parasite infection in fish will not only reduce the direct damage caused by the parasites, but also reduce fish mortality due to bacterial co-infection.
(Editor’s Note: This article was originally published in the July/August 2015 print edition of the Global Aquaculture Advocate.)
Authors
-
De-Hai Xu, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Unit
990 Wire Road
Auburn, Alabama 36832 USA[118,111,103,46,97,100,115,117,46,115,114,97,64,117,120,46,105,97,104,101,100]
-

Craig Shoemaker, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Unit -
Benjamin LaFrentz, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Unit
Related Posts
Health & Welfare
Economic impacts of aquatic parasites on global finfish production
Obligate and opportunistic parasites play a critical role in determining the productivity, sustainability and economic viability of global finfish aquaculture enterprises. Without stringent and appropriate control measures, the impacts of these pathogens can often be significant.

Health & Welfare
Parasite treatment reduces F. columnare infection in tilapia
The authors conducted a study to evaluate whether treatment of Trichodina-parasitized tilapia with formalin would improve fish survival and reduce F. columnare infection. Tilapia not treated with formalin showed significantly higher mortality than treated fish.

Health & Welfare
Sizing up TiLV and its potential impact on tilapia production
An international research effort has commenced to find a solution for Tilapia Lake Virus (TiLV), a contagion causing high rates of mortality in farmed and wild tilapia stocks in Israel, Colombia, Ecuador, Egypt and Thailand.

Responsibility
Addressing safety in Latin America’s tilapia supply chain
Over the last decade, the experience gained by many tilapia farmers combined with proficient programs implemented by local governments have significantly improved tilapia production in various Latin American countries like Colombia, Mexico, Ecuador and other important tilapia producers in the region.


