Maintenance of the developed clonal groups required almost no additional costs

Fish populations with good growth performance and the highest possible phenotypic uniformity are preferred by the aquaculture industry, to reduce labor and use of resources in the production and processing of products. One approach to improve these characteristics is the reduction of genetic variability by artificial production of clonal lines and use of these isogenic fish or isogenic crossbreds from two clonal lines for commercial production.
Homozygous clones
In fish, external fertilization and embryonic development allow the development of homozygous clones in only two generations through the use of induced gynogenetic or androgenetic reproduction. Whether a breeding strategy including clones or clone crosses is relevant for practical applications depends on the performance of the clone crosses, as well as the cost-effectiveness of the development and maintenance of the clonal lines.
Clonal lines of Nile tilapia
In recent years, a research group at the University of Göttingen’s Institute of Animal Husbandry and Genetics in Göttingen, Germany produced clonal lines of Nile tilapia (Oreochromis niloticus), one of the most important species in commercial aquaculture. Although clones have also been developed in other fish species, limited data have been published on their reproductive and growth performance in comparison to normal, genetically variable controls.
Development, reproduction
During the first step, homozygous O. niloticus were produced through “late” heat shock treatments at 41 degrees-C for 6 minutes, applied 25-35 minutes after egg activation with sperm that was genetically inactivated by ultraviolet irradiation. Six homozygous females produced through this mitotic gynogenesis procedure were fertile and served as clone mothers.
During the second step of clone development, eggs from these homozygous females were artificially stripped and given an “early” heat shock treatment (41 degrees-C for 4 minutes), 4.5 minutes after egg activation with genetically inactivated sperm (meiotic gynogenesis).
The clonal status of the resulting individuals was verified by gene marker studies. To continue the clonal lines, clonal females were again reproduced by meiotic gynogenesis.
Reproductive performance
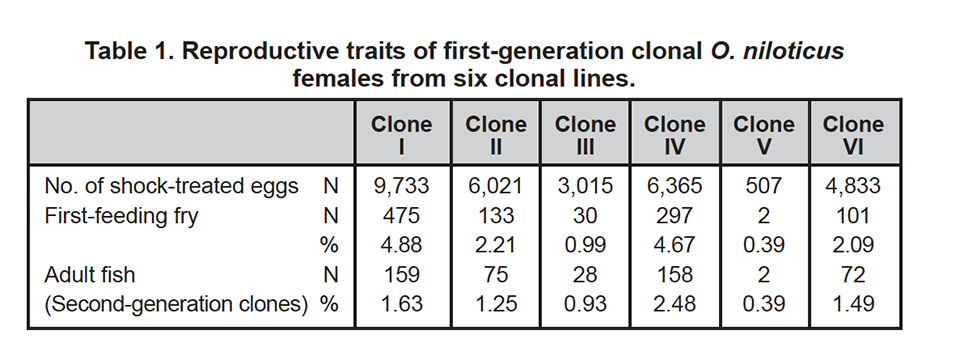
Table 1 provides data on the reproductive performance of females belonging to the six developed clonal lines. Compared to nonclonal control groups with a mean value of 43.9 percent for first-feeding fry, the tested O. niloticus clones were much more difficult to reproduce.
The treatment for gynogenetic reproduction seemed to be responsible for the reduced survival rates within clonal groups. Also, conventionally fertilized eggs from clonal females not exposed to shock had mean survival values as low as 11.2 percent for first-feeding fry, which indicated the reduced quality of eggs from clonal females.
Growth performance
Clonal and control groups were incubated and reared under identical conditions at 28 degrees-C. On day 20 after hatching, fish selected for growth performance testing were transferred into 80-liter glass aquaria. Depending on the number of available clonal fish per egg batch, each aquarium was stocked with up to 80 individuals.
Control groups were reared at corresponding stocking densities. As all gynogenetic clonal fish were females, all female control groups were used for the growth comparisons.
All groups were fed three times a day ad libitum with commercial pelleted feed. Fish were individually weighed on day 132 after hatching. Differences in mean body weights and variances of body weights between groups were analyzed statistically.
Table 2 presents body weight data obtained from clonal groups and corresponding controls. Differences in weight between clonal groups and controls were not as marked as they were for reproductive traits. Altogether, clonal groups showed slightly lower growth compared to controls, but these differences were insignificant.
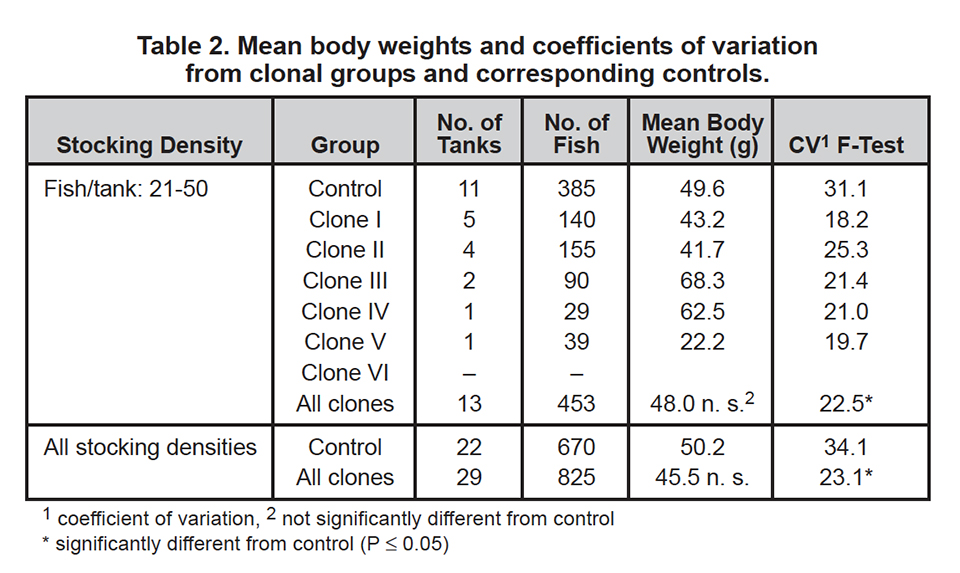
For tested groups with a stocking density of 21 to 50 individuals per tank, clones III and IV showed better growth than controls. All clonal O. niloticus groups had lower variabilities for body weight than the control groups, probably due to their genotypical uniformity. Maternal and environmental influences seemed to be responsible for the remaining variability observed within clonal groups.

Conclusion
This study demonstrated the difficulties of development and reproduction of clonal O. niloticus lines through induced gynogenesis. However, maintenance of the developed clonal groups required almost no additional costs and care, because their growth performance was comparable to, and in some cases better, than control groups.
It remains to be determined if continuation of clones by natural spawning of females with sex-reversed functional clonal males will be easier than through gynogenetic reproduction. From results of ongoing diallel crosses between the developed clonal lines, the Institute of Animal Husbandry and Genetics will determine if the development and continuation of clones in O. niloticus is worthwhile for practical applications in breeding programs.
(Editor’s Note: This article was originally published in the February 2002 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Andreas Müller-Belecke, Ph.D.
Institute of Animal Husbandry and Genetics
University of Göttingen
Albrecht-Thaer-Weg 3
D-37075 Göttingen, Germany[101,100,46,103,100,119,103,64,53,101,108,108,101,117,109,97]
-
Gabriele Hörstgen-Schwark, Ph.D.
Institute of Animal Husbandry and Genetics
University of Göttingen
Albrecht-Thaer-Weg 3
D-37075 Göttingen, Germany
Related Posts

Health & Welfare
Genetic screening in Nile tilapia
Both microsatellite and AFLP marker analyses can provide adequate screening of genetic diversity in tilapia stocks at a low cost.
Health & Welfare
Diagnostic profile of Belize Taura Syndrome Virus
Shrimp farming in Belize, Central America, was hard hit by Taura Syndrome Virus (TSV, now known as TSV serotype A) in 1996 and a re-emergence in 2002.
Health & Welfare
Rapid test detects NHP in penaeid shrimp
A rapid lateral-flow immunoassay using monoclonal antibody combinations can identify necrotizing hepatopancreatitis (NHP) infection in less than 15 minutes.

Health & Welfare
Perspectives for red-banded sea bream culture
Red-banded sea bream could be farmed in systems designed for other sparid species. The authors’ facilities have achieved spontaneous spawning.


