High-value species has significant culture potential

The red-banded sea bream (Pagrus auriga) is a commercially important sparid species in Spain with significant culture potential. Its culture is feasible in production facilities with other sparids such as the gilthead sea bream or even sea bass. The culture of this species could also be favored by its 20 to 30 euros per kg price in the market, which is much higher than the 5 to 6 euros per kg offered for gilthead sea bream.
Captive reproduction
Redbanded seabream are protogynous hermaphrodites whose sexual inversion occurs when their size is 55 to 65 cm and 3 to 5 kg. In captivity, the spawning season occurs from September to May in Spain. The authors’ facilities have achieved spontaneous spawning periodically since 2002.
During the spawning periods, water temperatures fluctuated 12 to 25 degrees-C. However, the highest numbers of eggs were detected routinely when the temperature ranged 19 to 22 degrees. Little or no egg batches were observed at temperatures lower than 15 degrees. The highest egg production occurred in October. A total of 5 million eggs were obtained in 2001, whereas the egg production increased to 35 million in 2005.
The mean average number of eggs per spawning was 105,674 ± 124,566 with a mean buoyancy percentage of 61 ± 18 percent. Experimental results have shown greater larval survival and total length at five days after hatching (DAH) at optimal egg incubation temperatures of 15 to 18 degrees-C.
Larval rearing
Larval rearing is a critical stage in redbanded seabream. Different variables have been studied in order to get better larval survival and growth rates. The authors have evaluated the effects of light intensity, diets, ontogeny of digestive enzymes and temperature.
To elucidate the roles of light intensity and diets on growth rate, larvae were reared under light sources of 400 or 800 lux, and fed live prey or an inert diet. Results indicated that larvae reared at 800 lux and fed live prey exhibited higher specific growth rates (SGRs) (17.6 and 16.5 percent daily, respectively). When these values were compared with those of other sparids, the authors determined that redbanded seabream fed live prey had growth rates up to sixfold higher than those of gilthead seabream and twofold higher than those of red porgy.
Regarding digestive capacity, the enzymatic activities for the main digestive enzymes during larval development to 40 DAH were also characterized. Trypsin activity increased progressively until 25 DAH. Thereafter, the activity kept constant at a high level of approximately 40 uf per individual. Similarly, chymotrypsin activity also increased with age, although the increase was faster during the first 10 days. Amylase activity increased in a continuous fashion during larval development, whereas esterases increased almost two orders of magnitude from three to 40 DAH.
Water temperature plays a key role in larval growth rates. A positive correlation between temperature and growth rate was identified. Larvae at 17 degrees-C exhibited a specific growth rate of 15.8 percent/day, whereas at 22 degrees the values rose to 22.5 percent daily. However, these higher growth rates were associated with lower larval survival – 5 percent at 17 degrees and only 2 percent at 22 degrees-C. In addition, no influence on growth or survival was identified when larvae were fed artemia at 15 DAH or 20 DAH.
Nursery, grow-out studies
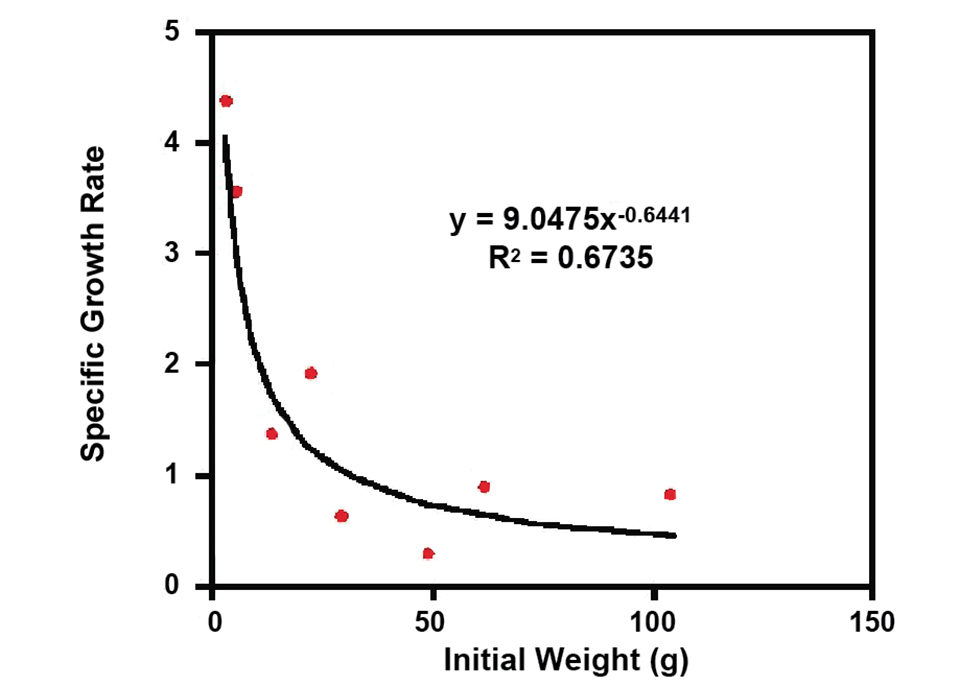
The authors recently carried out a four-month nursery experiment with fish born in captivity. Survival rates up to 98 percent were excellent. The average specific growth rate ranged 0.9 to 3.3 percent per day (Fig. 1). Initial grow-out results were highly promising with growth rates similar to those described for gilthead seabream. The best specific growth rate values ranged 0.8 to 2.1 percent per day in tanks and 1.9 to 4.4 percent per day in cages. During broodstock grow-out work carried out 2001 to 2007, SGRs varied 0.01 to 0.14 percent per day.
Diseases
Due to the intensive production technology employed for redbanded seabream, some disease outbreaks have been recorded. The most common symptoms were exophthalmia, hemorrhages at the base of fins and, in some cases, corneal opacity, mucus-covered and pale gills, and necrotic foci in the liver and splenomegalia. Mortality ranged 10-20 percent with most of the outbreaks registered in the month of June.
A total of 11 (Photobacterium damselae) subspecies damselae isolates have been identified from six outbreaks between 2003 and 2006. The isolates revealed a high polymorphism at the biochemical level. All of them were sensitive to flumequine, although some showed resistance to oxytetracycline. Moreover, virological analyses have allowed the identification of nodaviruses in some asymptomatic specimens. Nevertheless, the pathological relevance of this discovery remains to be demonstrated.
In order to monitor the health status of redbanded seabream specimens, the authors developed an indirect ELISA assay using polyclonal antibodies. Data demonstrated the utility of this assay to measure the antibody levels after vaccination. In fact, preliminary analyses in animals inoculated with P. damselae ssp. damselae or (Vibrio anguillarum) bacterins exhibited higher immunoglobulin levels than controls. Also, approximately 7.4 percent of specimens had skeletal malformations, mainly lordosis, although developmental anomalies in the jaw and operculum have been recorded.
Seabream/porgy hybrids
Recently, an intergeneric hybrid between the red-banded sea bream and the phylogenetically closely related species red porgy (Pagrus pagrus) has been achieved. Red porgy overlap the spawning season of redbanded seabream from December to April.
To test the hypothesis of spontaneous hybridization, the authors placed 27 red porgy breeders together with 121 redbanded seabream broodstock in the same tank. During February, March and April, all the larvae were pooled and cultured. After weaning, the red-banded sea bream and red porgy fry were clearly identifiable. Moreover, in the batch from March, some other animals sharing phenotypic characteristics with both species were detected.
The percentage of hybrids was around 5-6 percent both in 2004 and 2005. Molecular analysis based on mitochondrial DNA, satellite and nontranscribed spacer sequences confirmed that those animals were hybrids between female P. pagrus and male P. auriga. The reciprocal cross was not identified. At this moment, the authors do not know if these reciprocal crosses are viable or not.
New experiments using artificial cross-fertilization are ongoing. The achievement of both hybrids separately will allow evaluation of the differences in larval survival as well as growth rate in both hybrids and their parents.
In addition, specific microsatellite markers for P. auriga have been developed. These new molecular markers will provide useful tools to assess the genetic variability and structure of cultured populations. The development of new progeny assignment tests will allow establishing the reproduction pattern of each breeder in a communal tank and its particular contribution to the offspring.
Perspectives
Some companies have shown interest in the commercial-scale culture of this species. In Andalucia and Canarias, Spain; Turkey and Israel; there is some marketing of 0.3- to 0.8-kg fish. However, the further development of an aquaculture industry for this species will require more information about the control and synchronization of reproduction using photoperiod and thermoperiod, the improvement of survival rates during larval development, the development of species-specific diets, and greater knowledge of the diseases that affect this species and the genetic variability of the stocks in captivity.
(Editor’s Note: This article was originally published in the May/June 2008 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Salvador Cardenas
IFAPA Centro El Toruño
Junta de Andalucia
P.O. Box 16
El Puerto de Santa María
Cadiz, Spain[115,101,46,97,105,99,117,108,97,100,110,97,101,100,97,116,110,117,106,64,115,97,106,111,114,46,115,97,110,101,100,114,97,99,46,114,111,100,97,118,108,97,115]
-
Manuel Manchado
IFAPA Centro El Toruño
Junta de Andalucia
P.O. Box 16
El Puerto de Santa María
Cadiz, Spain
Tagged With
Related Posts

Aquafeeds
A look at phospholipids in aquafeeds
Phospholipids are the major constituents of cell membranes and are vital to the normal function of every cell and organ. The inclusion of phospholipids in aquafeeds ensures increased growth, better survival and stress resistance, and prevention of skeletal deformities of larval and juvenile stages of fish and shellfish species.

Health & Welfare
Dietary acidification in aquaculture
Much of the chemical breakdown of foodstuffs begins in the stomachs of animals through acidification. Effective use of digestive biology is a goal in aquaculture, so dietary acidifiers have been gaining interest in recent years.

Health & Welfare
Botanical extracts improve productivity of shrimp, pangasius
In a study with pangasius, dietary administration of a blend of botanical extracts improved performance, reducing intensity of gill parasite infestation.
Health & Welfare
Computer modeling helps grow fish
For farmers, computer modeling helps analyze crop production and environmental effects. For managers, it assists with carrying capacity assessments and licensing issues.


