Cyanobacteria cause dissolved-oxygen fluctuations

Phytoplankton form the base of the food web in aquaculture ponds, but the natural abundance of phytoplankton is not sufficient to provide desired levels of shrimp and fish production. Fertilizers increase the natural fertility of ponds and allow greater yields. Many producers, however, have switched to feed-based aquaculture to increase production beyond that possible with fertilizers.
Natural productivity is still important in ponds with feeding, especially soon after stocking, because young postlarvae and fingerlings cannot use feed as efficiently as older animals. Some aquaculturists, and particularly shrimp producers, evaluate the abundance and taxonomic composition of phytoplankton communities in pond waters. They also apply various treatments in attempts to control the abundance and composition of the phytoplankton communities.
Algal propagules are ubiquitous – fill a new pond with water and many phytoplankton species will find their way into it. Phytoplankton growth is regulated by water temperature, solar radiation, pH, turbidity and nutrient concentrations. Acidic pond water normally is treated with liming materials to increase pH. Turbidity caused by suspended soil particles usually settles from water held in ponds, and nutrients are supplied by fertilizers and feeds. Aquaculture ponds typically have ideal conditions for the growth of phytoplankton.
Density, species composition
The density of phytoplankton blooms is strongly dependent upon the availability of nutrients, especially nitrogen and phosphorus. Intensive aquaculture ponds with large inputs of feed usually have an abundance of phytoplankton, and phytoplankton blooms also reach fairly high densities in semi-intensive ponds.
The species composition of phytoplankton blooms is highly variable and can change rapidly over time. Many have observed that adjacent aquaculture ponds receiving similar management inputs seldom have water of equal clarity or color. Differences in the appearance of pond waters result from differences in the composition and density of phytoplankton blooms.
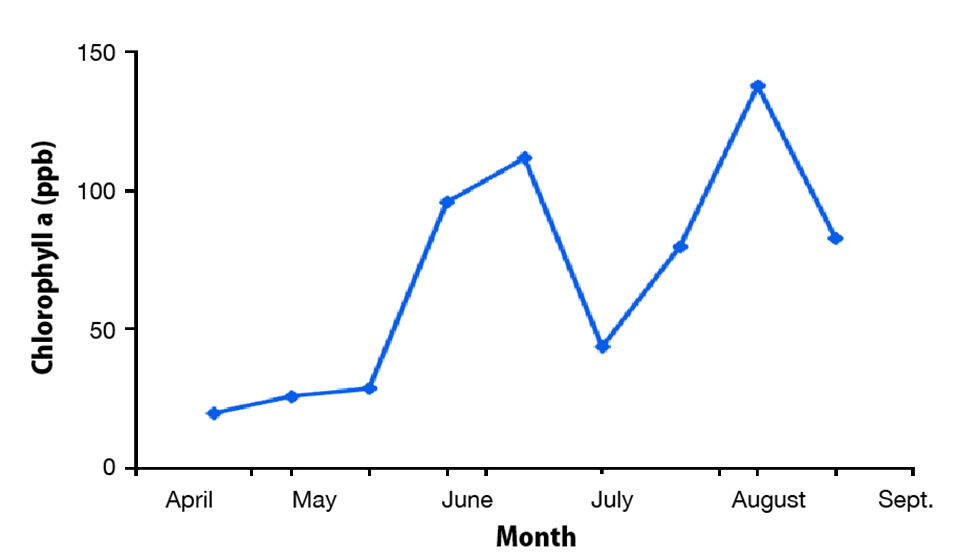
A pond might have a phytoplankton bloom consisting primarily of various species of green algae, but within a few weeks, the phytoplankton community might be made up almost entirely of a single species of blue-green algae. Alternatively, the green algae bloom in a pond might persist, but the species in the bloom might change during a period of a few weeks. The total abundance of phytoplankton also waxes and wanes (Fig. 1), even when nutrient inputs are relatively constant.
Blue-green algae
Some phytoplankton species are more desirable than others in aquaculture ponds. Blue-green algae, called cyanobacteria by some, are particularly troublesome. Dense blooms of blue-green algae, like those of other algae, cause wide daily fluctuations in dissolved-oxygen concentration. Mechanical aeration has provided a means of avoiding excessively low dissolved-oxygen concentrations at night in intensive aquaculture ponds.
Blue-green algae often float to the surface and form scums during calm weather. These scums absorb heat and elevate water surface temperatures. They also often drift into corners of ponds, where they die and result in localized water and soil quality deterioration.
Blooms of blue-green algae also tend to die suddenly. Dense phytoplankton blooms consisting of a single species of blue-green algae and calm, warm weather with intense solar radiation are conditions that favor die-offs of phytoplankton. Massive die-offs can lead to dissolved oxygen depletion despite the operation of mechanical aerators.
In addition to causing water quality problems, some species of Oscillatoria, Anabaena, Microcystis, Lyngbya, Aphanizomenom and a few other blue-green algae genera are notorious for producing odorous compounds that when absorbed by fish and shrimp cause off-flavors in their flesh.
Once off-flavor compounds disappear from the water, they undergo natural depuration from the flesh of culture species. Ponds can be treated with copper sulfate at a rate equal to 1 percent of the total alkalinity to kill blue-green algae. Following elimination of the source of odorous compounds, culture animals frequently, but not always, return to normal flavor within one or two weeks. In coastal ponds, water exchange sometimes can be used to flush off-flavor-producing algae from ponds.
Factors favoring blue-green algae in ponds are high concentrations of nutrients and pH above 8.3. High pH favors these algae because they are much more competitive than other algae for the low concentration of available inorganic carbon at high pH. Aquaculture ponds are ideal for blue-green algae because nutrients are abundant and algae grow rapidly. This reduces carbon-dioxide concentrations, raises pH and further favors dominance by the algae.
Research also has shown that blue-green algae can release algicidal substances into the water that are toxic to other types of algae. In the United States, 75-80 percent of channel catfish ponds have phytoplankton communities dominated by blue-green algae in late summer and early fall. Blue-greens occur in brackishwater ponds, but their abundance tends to be greatest when salinities are below 10 ppt.
Some species of blue-green algae produce toxins that can kill fish and shrimp. The most famous toxic algae are species of the genera Prymnesium and Chrysochromulina. These algae are mainly marine, but some species of Prymnesium can thrive in inland waters with salinities above 2 ppt.
Fish kills often are caused by blooms of golden algae, P. parvum. Recently, this species caused mortality and poor growth of shrimp in inland ponds with salinities of 2 to 5 ppt in Alabama, USA. Other toxic algae include some species of dinoflagellates (red tide is caused by the dinoflagellate Ptychodiscus brevis), a few marine diatoms and marine chloromonads.
In freshwater ponds, copper sulfate is used to kill toxic algae. Some producers treat water with 2 to 4 mg potassium permanganate/l to oxidize algal toxins remaining in the water. In coastal ponds, it sometimes is possible to flush toxic algae from ponds by water exchange.
Bloom management
Aside from blue-green algae and toxic algae, most other planktonic algae are not particularly troublesome in aquaculture ponds. Green algae are considered most desirable in freshwater ponds. Many managers of brackishwater or seawater ponds for shrimp culture prefer blooms of diatoms, for these algae are considered good natural food for shrimp.
There are no reliable methods for maintaining blooms of green algae in freshwater ponds. Although ponds can have green algae early in the crop, phytoplankton dominance often later shifts to blue-green algae.
Research conducted in water confined within large plastic enclosures in a eutrophic lake showed the blue-green algae were replaced by green algae when carbon dioxide was introduced into the enclosures. As a result, some shrimp farmers add manures or molasses to ponds to stimulate microbial production of carbon dioxide to lower pH and discourage blue-green algae. The benefit of this procedure has not been proven through careful study.
Shrimp farmers have been successful in stimulating diatom production in brackishwater or seawater ponds by fertilizing with a wide nitrogen:phosphorus ratio – 10 to 20 times as much nitrogen as phosphorus. Many shrimp producers believe that high diatom abundance is favored by fertilization with nitrate and silicate. A commercial fertilizer from Chile specifically formulated for increasing diatom abundance in shrimp ponds contains both sodium nitrate and silicate. Research findings also suggest that the addition of iron to seawater should stimulate diatoms, but no studies verify the efficacy of this treatment in shrimp ponds.
In attempts to reduce the abundance of phytoplankton, calcium, iron and aluminum compounds have been applied to ponds to precipitate phosphorus from the water. The rationale behind this method is that removal of phosphorus lowers the photosynthesis rate, increases carbon dioxide availability and lowers pH – a sequence of events unfavorable to blue-green algae growth. These treatments have seen some success in research but are seldom used by commercial producers.
Algae evaluation
Many shrimp farmers routinely count the abundance of different genera of algae in pond waters. Aside from fertilizing to encourage diatoms or treating off-flavor-producing algae and toxic algae with copper sulfate, little can be done to control the composition of algae communities. It is doubtful that the tremendous effort involved in algae enumeration is worthwhile. However, some evaluation of algal communities is desirable for determining if potentially toxic species or species capable of producing odorous compounds are present.
(Editor’s Note: This article was originally published in the January/February 2009 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Health & Welfare
Ammonia toxicity degrades animal health, growth
Ammonia nitrogen occurs in aquaculture systems as a waste product of protein metabolism by aquatic animals and degradation of organic matter, or in nitrogen fertilizers. Exposure can reduce growth and increase susceptibility to diseases in aquatic species.

Responsibility
Appraising pond liners for shrimp culture
The use of plastic-lined ponds by shrimp farmers can significantly improve production efficiency, support more production cycles per year, and higher mechanical aeration rates and stocking densities. The capital cost of lining ponds can be very significant, so a thorough feasibility analysis is recommended when considering this production tool.

Responsibility
Carbon-nitrogen ratios in pond fertilization and biofloc systems
Prof. Claude Boyd on the importance of carbon-nitrogen ratios for pond fertilization and biofloc systems, and the relevance of precise carbohydrate inputs.

Responsibility
Assessing groundwater quality in aquaculture
Those interested in using groundwater for aquaculture should perform a thorough chemical analysis of the water. Several problems related to groundwater use in hatcheries and holding or transport vessels can be alleviated by degassing or aeration.

