Researchers note higher survival and histological signs of hepatopancreas regeneration in Vietnam trials with INVE

Early mortality syndrome (EMS) is a management syndrome. A combination of adverse factors in nutrition, biosecurity, host physiology, and especially microbial management leads to a situation in which opportunistic pathogens such as Vibrio parahaemolyticus can bloom and dominate the microbiota around and inside the shrimp. When additional virulence factors such as colonization of the stomach and toxin production are added to this setting, diseases such as acute hepatopancreas necrosis (AHNPD) will cause severe losses.
INVE Aquaculture has already been working on this issue for more than a decade, in the context of “traditional” vibriosis. Hence it was a logical step to extrapolate our established pro- and metaphylactic treatments to this new variant of Vibrio. When applying our probiotics in the field, as part of a holistic intervention protocol, a significant amount of empirical data of the beneficial action of Bacillus probiotics during shrimp culture has been collected. In this study our objective was to measure the effects of Sanolife probiotics in a standardized AHPND challenge model under controlled laboratory conditions.
Test shrimp
Pacific white shrimp (Litopenaeus vannamei) were bred and nursed at the shrimp hatchery and nursery of the College of Aquaculture and Fisheries, Can Tho University. Shrimp stocks were under surveillance for WSSV, YHV (IQ2000 YHV/GAV) and AHPND Vibrio to maintain SPF status.
For this study, shrimp around PL20-25 stage, with an average body weight around 1 g were used, the age and size which is most affected by EMS/AHPND under culture conditions. Natural seawater was used throughout the experiments, sterilized and diluted to 25 g/L, a typical salinity for P. vannamei grow-out. This study was originally published in Aquaculture Asia Pacific 11(6):14-17.
Bacteria
We designated the bacterial strain used in this study as LTS14. It was originally isolated from shrimp diagnosed with AHPND (histo)pathology in Vietnam in May 2014 and stored at -80°C in TSB supplemented with 1.5 percent NaCl and 25 percent glycerol. The bacteria were identified as V. parahaemolyticus, by green colonies on TCBS, conventional API 20E biochemical tests and PCR with LTH primers. Additionally, the isolate was positive on PCR with AP3 primers.
Prior to the study, the virulence of LTS14 was extensively evaluated by in vivo challenges, and compared to other strains. The challenge dose was fine-tuned in order to obtain a reproducible sub-acute LD50-60 mortality curve (Figure 1).
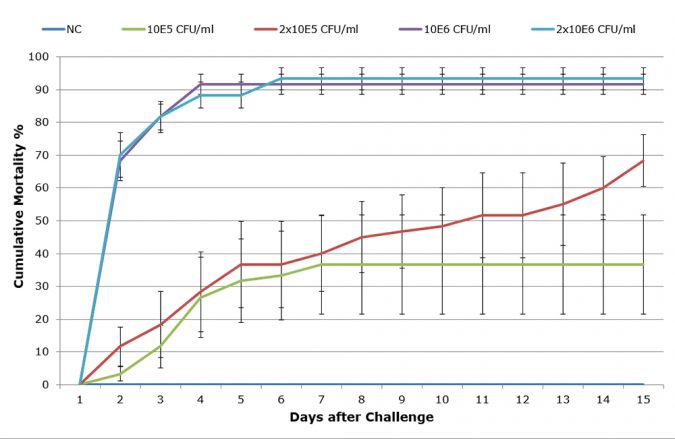
Challenge
Bacterial cultures were grown 24 hours in TSB supplemented with 1.5 percent NaCl at 28°C. Based on the standard curve determined for the strain, the bacterial suspension was diluted in sea water to an OD corresponding to 108 cells ml-1. Shrimp were immersed for 15 minutes in this bacterial culture with continuous aeration and then both bacterial solution and shrimp were transferred to aquaria containing sea water, reducing the bacterial concentration to 105, 2×105, 106, 2×106 cells/mL. No water was exchanged until two days after the challenge, from which point 20 percent of water was renewed daily.
Experimental design
Experimental shrimp were stocked at a density of 30 individuals per aquarium with 30 liters of water, continuous aeration and water parameters held constant at 29±1°C, pH 7.7±2, NH3 <0.1 mg/L and DO 4 mg/L by daily water exchange.
The following five treatments in triplicate were compared:
| Treatment description | Challenge with V. parahaemolyticus | |
| Negative control (NC) | – | – |
| Positive control (PC) | – | + |
| Antibiotic control (AB) | doxycycline 2g kg-1 feed | + |
| Sanolife PRO-2 (PRO-2) | 10g kg-1 feed | + |
| Sanolife PRO-W (PRO-W) | 5 mg l-1 | + |
Feed applications were top-coated with every ration and the Sanolife PRO-W water application was added to the water of the aquarium once per day. The dose of Sanolife PRO-2 was 2×108 CFU Bacillus per g feed and the dose of Sanolife PRO-W was 2.5×105 CFU Bacillus per ml water. Apart from the NC, all shrimp were challenged with 2×105 CFU/mL of LTS14, and clinical follow-up was performed for 15 days after challenge.
The evaluation of the treatments was based on statistical comparison of: (1) the severity and time of onset of clinical signs; (2) the cumulative mortality; and (3) the severity of score on histopathology.
Severity of clinical signs AHPND
Clinical signs such as anorexia, lethargy and pale coloration of the body and hepatopancreas were observed in 75 percent of the animals in the positive control group as early as 24 hours after the challenge. Shrimp in the AB and Sanolife PRO-W groups also demonstrated AHPND symptoms, but with less pronounced anorexia, and in a reduced group of animals (50 percent). Less than 20 percent of shrimp receiving Sanolife PRO-2 were recorded with AHPND symptoms, and with a significant delay of 72h after challenge. Representative photos of gross signs are shown in Figure 2.
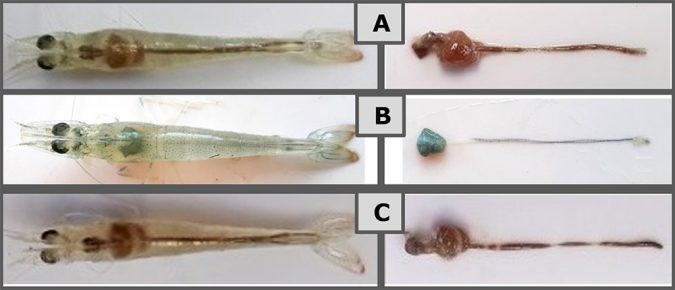
Reduced mortality
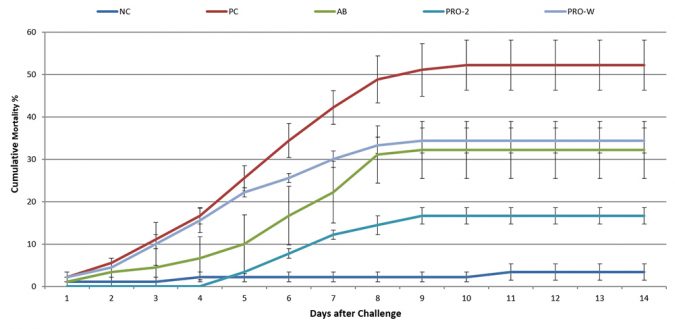
Mortality started in the PC group 1 dpi (days post-inoculation) and reached a cumulative mortality of 52±10 percent after 10 days (Figure 3). In AB and Sanolife PRO-W groups, mortality also started at 1 dpi and cumulative mortality attained 32±12 percent and 34±5 percent respectively. A delay in mortality of 4 days was noted for the Sanolife PRO-2 group, with cumulative mortality stopping at 17±3 percent after 9 days. The Sanolife PRO-2 result was statistically significantly lower than the PC, but also still higher than the NC 3±3 percent.
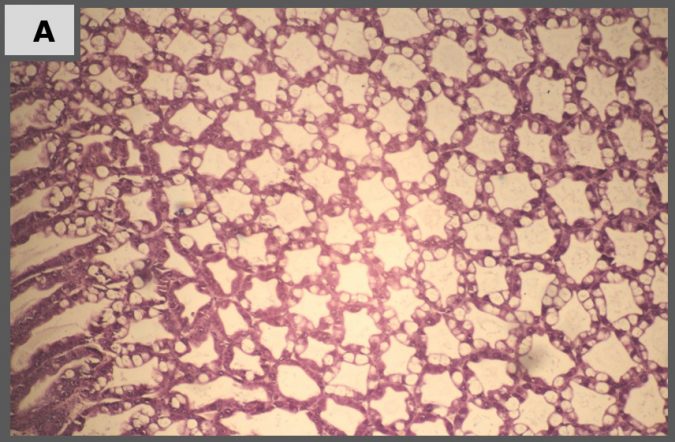
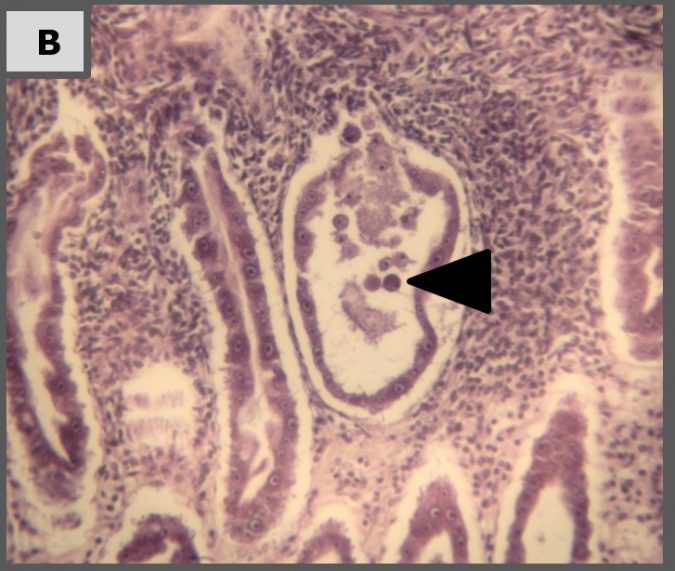
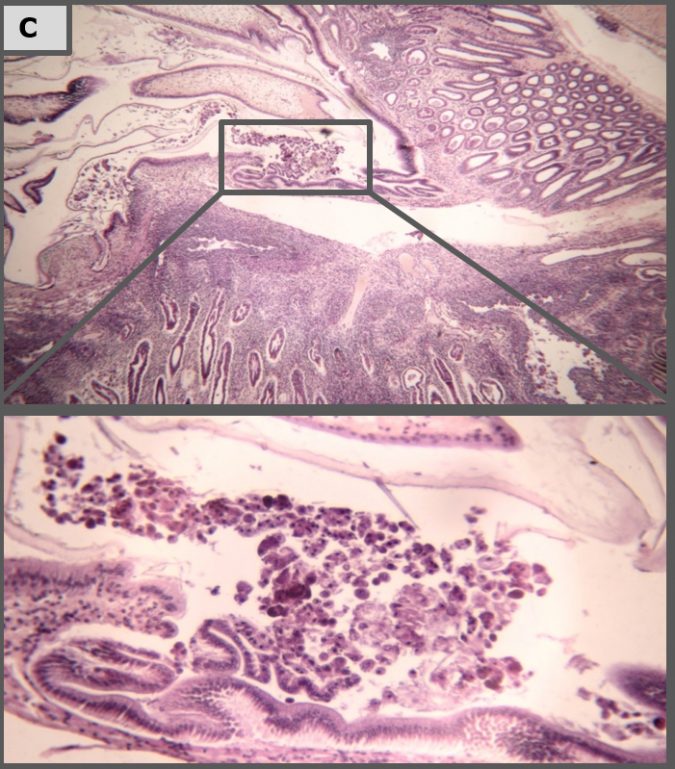
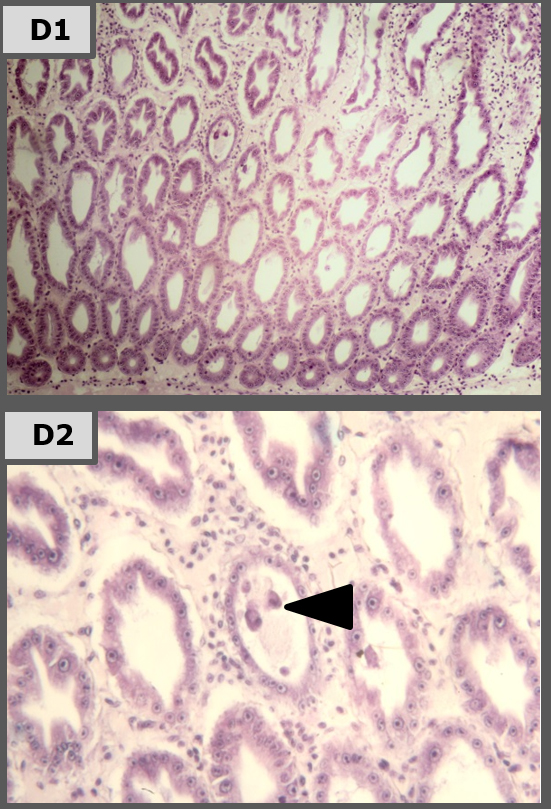
Histopathology
Representative images of histopathological analysis of shrimp in the different treatments.
Perspectives
The AHPND challenge model developed and standardized for this study resulted in a mortality curve of the positive control which reaches its maximum after several days, and does not wipe out all the inoculated shrimp. This is more in line with AHPND outbreaks in shrimp farms and also offers a better chance for evaluating possible therapeutic interventions than many reported challenge models employing impossibly high bacterial concentrations and resulting in hyper-acute mortality.
The results of our laboratory study show that Sanolife PRO-2 and Sanolife PRO-W probiotics treatments by themselves have beneficial effects, such as higher survival and histological signs of hepatopancreas regeneration. However, similar to antibiotic treatments, probiotic treatments are not sufficient to completely protect shrimp from disease. For this, a holistic approach is needed, supporting and correcting the rearing system and the shrimp’s health status on all levels.
Authors
-

Dang Thi Hoang Oanh, Ph.D.
Head, Department of Aquatic Pathology
College of Aquaculture and Fisheries
Cantho University
3-2 Street, Ninhkieu District
Cantho City, VIETNAM[110,118,46,117,100,101,46,117,116,99,64,104,110,97,111,104,116,100]
-

Mathias Corteel, Ph.D.
R&D Engineer
INVE Aquaculture,
Hoogveld 93, 9200 Dendermonde
BELGIUM[109,111,99,46,101,114,117,116,108,117,99,97,117,113,97,101,118,110,105,64,108,101,101,116,114,111,99,46,109]
-

Olivier Decamp, Ph.D.
Product Manager Health
INVE Aquaculture
Hoogveld 93, 9200 Dendermonde
BELGIUM[109,111,99,46,101,114,117,116,108,117,99,97,117,113,97,101,118,110,105,64,112,109,97,99,101,100,46,111]
Tagged With
Related Posts

Health & Welfare
Limited decomposition enhances PCR detection of AHPND Vibrio in shrimp
A study confirmed the utility for improved polymerase chain reaction (PCR) detection of the Vibrio bacteria that cause acute hepatopancreatic necrosis disease (AHPND) in asymptomatic shrimp by permitting the shrimp to expire and decompose for several hours prior to preservation and PCR processing.

Health & Welfare
PCR methods characterize AHPND V.p isolates
In analyzing the plasmid sequence from the whole genome sequences of AHPND V. parahaemolyticus (V.p) isolates, researchers identified a clear geographical variation within the plasmid, and developed PCR methods to characterize AHPND V.p isolates as either Mexico-type or Southeast Asia-type.

Health & Welfare
Focus on gut health increases aquaculture productivity
Increased attention to nutrient digestibility and gut health are two important focus areas for technological development of manufactured feeds in the aquaculture industry. Both laboratory and field studies have demonstrated the potential benefits for aquaculture productivity and the economics of specific feed additives developed to stimulate the establishment of a healthy gut microflora.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

