Thermal treatments inactivate pathogens in salmon, shrimp

As the United States continues to consume increasing volumes of seafood, the country is entering a new era in which dwindling natural fisheries resources are forcing regulatory agencies to develop a more holistic approach to seafood safety and natural marine resource issues. Aquaculture species now contribute approximately 15-20 percent of the U.S. seafood supply.
Although the United States has one of the safest food supplies in the world, the Centers for Disease Control (CDC) estimates that as many as 76 million people in the U.S. are stricken with food-borne illnesses from all foods each year, of which 300,000 are hospitalized and 5,000 die. Food safety must therefore be an integral part of the farm-to-table food continuum. Microbial food safety risks can be reduced by good sanitation during processing and storage, and proper cooking of the final products.
According to the CDC, the most commonly reported proliferation factor of pathogens in food is allowing foods to remain at room or warm outdoor temperature for several hours. Insufficient cooking and the public’s preference for more raw-like products can also lead to problems.
In addition, it is possible to produce food that appears to be cooked but has not reached lethality temperatures for the destruction of pathogens. This can be prevented if manufacturers provide consumers with recommended internal cooked product temperatures along with understandable cooking instructions.
Salmonella in seafood
The CDC summary of outbreaks of food-borne illness associated with seafood from 1998 to 2004 in Table 1 shows that Salmonella was the leading cause of bacterial-associated outbreaks and cases in non-molluscan seafood. It accounted for 10 outbreaks and 224 cases of food-borne illness associated with crustaceans, 14 outbreaks and 852 cases for finfish, and two outbreaks and 13 cases in molluscan shellfish. Contamination of seafood with Salmonella is usually associated with fecal contamination of harvest sites, but is also associated with cross contamination by food handlers and consumers, and undercooking of food products.
Jahncke, Outbreaks and cases of food-borne illness, Table 1
| Etiological Agent | Outbreaks (Total Cases) Crustaceans | Outbreaks (Total Cases) Finfish | Outbreaks (Total Cases) Molluscan Shellfish |
|---|---|---|---|
| Anisakis simplex | – | 1 (14) | – |
| Bacillus cereus | 1 (118) | 1 (3) | – |
| Campylobacter jejuni | – | 2 (140) | 1 (2) |
| Clostridium botulinum | – | 3 (8) | – |
| Clostridium perfringens | 1 (204) | – | – |
| Cyclospora cayetanensis | – | 1 (56) | – |
| Escherichia coli | 1 (12) | 1 (41) | – |
| Plesiomonas shigelloides | – | 1 (5) | – |
| Salmonella | 10 (214) | 14 (852) | 2 (13) |
| Shigella sonnei | 1 (2) | 1 (47) | 2 (6) |
| Staphylococcus aureus | – | 1 (3) | – |
| Vibrio cholerae | 1 (6) | – | 3 (8) |
| Vibrio fluvialis | – | – | 1 (2) |
| Vibrio parahaemolyticus | 9 (142) | – | 13 (507) |
| Multiple bacteria | 3 (38) | 1 (2) | – |
| Noroviruses | 7 (294) | 13 (668) | 17 (429) |
| Hepatitis A virus | 2 (14) | 1 (4) | – |
| Total known | 36 (1,044) | 41 (1,843) | 39 (967) |
| Unknown | 119 (921) | 207 (1,287) | 108 (661) |
| Total | 155 (1,965) | 248 (3,130) | 147 (1,628) |
Salmonella has been isolated at three U.S. coastlines at a prevalence rate of 7.4 percent. Danielle Brands and co-authors reported in 2005 that S. enterica serovar Newport, a human pathogen, comprised over 75 percent of the isolates. In 1998, a study conducted by the U.S. Food and Drug Administration (FDA) confirmed that farmed seafood was more likely to contain Salmonella than wild-caught seafood.
In this study, 124 samples of aquaculture seafood and 240 samples of wild-caught seafood were collected at the retail level and analyzed for the presence of Salmonella. Both imported and domestic seafood were sampled. Slightly over 6 percent of the aquaculture seafood contained Salmonella, while less than 1 percent of the wild-caught seafood had it.
As explained by Maxine Heinitz and co-authors in the May 2000 Journal of Food Protection, from 1990 to 1998, FDA collected and tested 11,312 imported and 768 domestic seafood samples for the presence of Salmonella, with nearly 10 percent of imported raw seafood and 1.3 percent of domestic raw seafood found positive for Salmonella. Three common Salmonella serotypes found on imported seafood were Salmonella Enteritidis, Salmonella Newport and Salmonella Typhimurium. Others included Salmonella Weltevreden, Salmonella Senftenberg, Salmonella Lexington and Salmonella Paratyphi-B.

Safe cooking parameters
To address food-borne illnesses associated with seafood, the National Advisory Committee on Microbiological Criteria for Foods (NACMCF) developed a document entitled “Determining Cooking Parameters for Safe Seafood for Consumers” in 2008. Conclusions of the NACMCF document included:
- There is a lack of thermal inactivation data for relevant pathogens in seafood products due, at least in part, to the wide variety of products available and the many methods of cooking applied to the products.
- Additional studies are needed to establish appropriate consumer-based time/temperature cooking parameters to ensure safety and maintain the sensory quality of seafood products.
- Cooking processes should be based on time and temperature parameters dependent on the thermal inactivation kinetics of the pathogens, heat transfer properties of the product and levels to which the pathogens must be destroyed.
Consumers, retailers and restaurants use numerous cooking methods to prepare seafoods. Yet cookbooks and other commercial resources offer cooking advice that provides primarily subjective measures for determining if seafood is properly cooked. The advice focuses mainly on quality aspects. Fish are “done” when the flesh becomes opaque, flakes and easily separates from the bones. For crustaceans, the meat becomes opaque and the shell color changes. For molluscan shellfish, the shells open. Such subjective measures of “doneness” are not science-based and may not yield safe products.
The report also stated that further guidance to consumers on how to properly cook seafood products to ensure safety and optimize quality is needed. Although FDA’s 2005 U.S. Food Code recommends minimum internal cooking temperatures for intact fish of 63 degrees-C or above for 15 seconds, for comminuted fish 68 degrees-C or above for 15 seconds and 74 degrees-C or above for 15 seconds for stuffed fishery products, these recommendations are not based on empirical studies conducted on seafood products.
Thermal inactivation, physical characteristics
To address the issues identified in the NACMCF document and the lack of specific data for seafood products, researchers at Virginia Tech are conducting work on the thermal inactivation of Salmonella in a variety of seafood products and applying mathematical models to determine decimal reduction (D) values and Z values.
A D value is the measure of heat resistance of a microorganism. It is defined as the time in minutes at a given temperature required to destroy 1 log cycle (90 percent) of the target microorganism. A Z value reflects the temperature dependence of the microorganism – the number of degrees needed to change a D value by a factor of 10. Small Z values indicate microorganisms are less heat-tolerant, while larger Z values indicate more heat tolerance.
In studies supported by funding from the National Fisheries Institute Fisheries Scholarship Fund and Virginia Sea Grant, Virginia Tech is also conducting research on consumer cooking methods to determine physical characteristics (firmness, cohesiveness, color, expressible moisture, soluble proteins, etc.) for seafood products using popular consumer cooking methods. In addition, researchers are measuring the physical characteristics relative to common culinary descriptors.
Salmonella inactivation studies
Thermal inactivation experiments have been conducted by the author using a mixture of Salmonella species inoculated into raw, homogenized farmed Atlantic salmon and white shrimp. The results of the thermal inactivation studies are shown in Figs. 1, 2 and 3.
Fig. 1 shows the thermal inactivation curve in minutes for Salmonella inoculated in salmon and then heated at 60 degrees-C. Fig. 2 shows the thermal inactivation curves for Salmonella inoculated in salmon and heated. Fig. 3 shows the thermal inactivation curves for Salmonella inoculated in shrimp and heated to several temperatures.
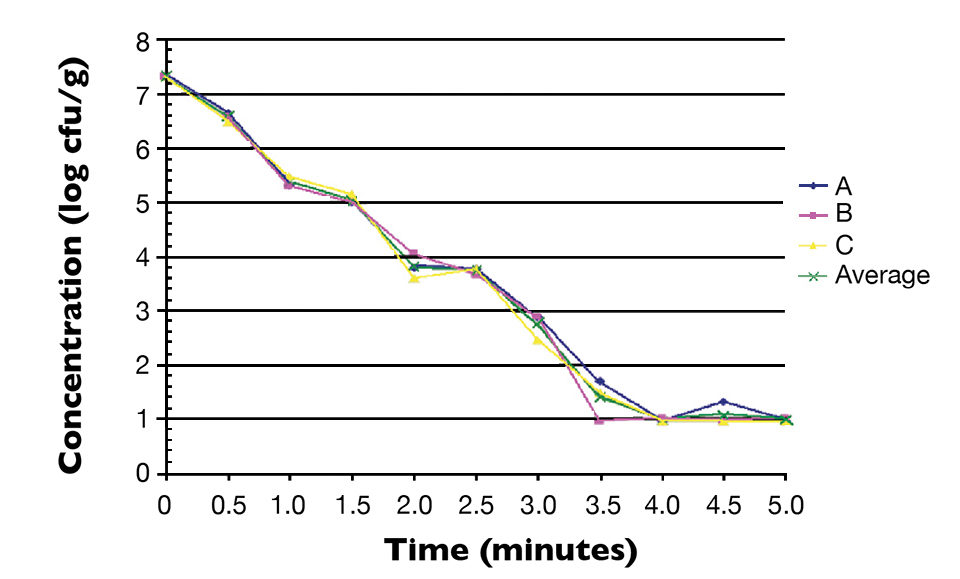
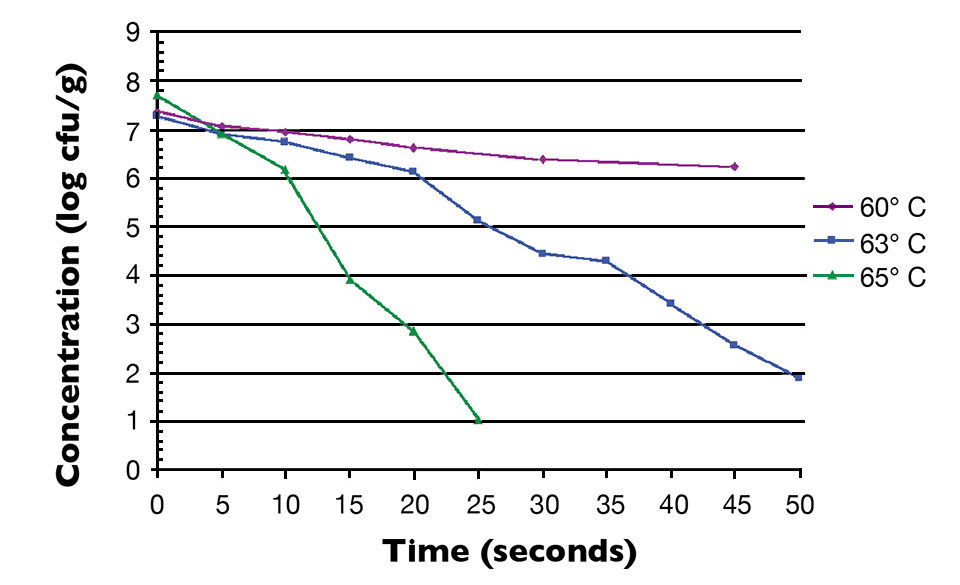
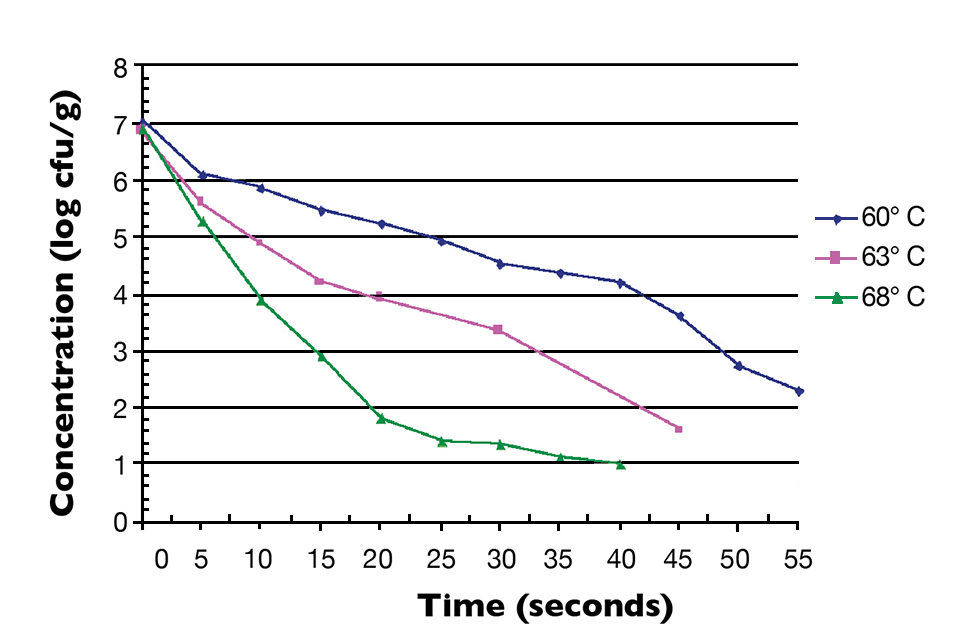
The results indicated D values for Salmonella in salmon of 38.0 seconds at 60 degrees-C, 9.0 seconds at 63 degrees and 3.6 seconds at 65 degrees-C. The Z value was 4.9 degrees-C. The calculated D values for Salmonella in shrimp were 15.0 seconds at 60 degrees-C, 7.8 seconds at 63 degrees-C and 3.2 seconds at 68 degrees-C, with a Z value of 12 degrees-C.
The calculated Z value for shrimp in these studies was higher than the Z values for Salmonella in other food products. Unlike the salmon, the shrimp were not able to be homogenized into a smooth paste. There were membrane components and a more lumpy consistency to the homogenate, which may have provided some thermal protection to the inoculated Salmonella.
A similar study by Merrill McPhearson and Sabrina Zywno also showed higher Z values for inoculated Salmonella in shrimp. They calculated a Z value of 11 degrees C and D values of 41 seconds at 60 degrees-C, 37 seconds at 61.4 degrees and 23 seconds at 62.8 degrees-C for S. enterica Serotype Weltevreden inoculated into brown shrimp (Penaeus aztecus). S. Weltevreden has also been shown to be a more heat-resistant Salmonella serotype.
Future research
Future research will include additional thermal inactivation studies using other Salmonella serotypes and seafood products. In addition, seafood products will be prepared by different cooking methods, and then the visual characterization and intensity of products at endpoints will be assessed by a trained panel using descriptive culinary terminology with the Sensory Information Management System. Color changes will be measured using a tristimulus colorimeter, while firmness, cohesiveness and expressible moisture changes will be measured using
a texture analyzer.
Editor’s Note: The authors’ research is supported by funding from the National Fisheries Institute Fisheries Scholarship Fund and Virginia Sea Grant.
(Editor’s Note: This article was originally published in the September/October 2009 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Michael Jahncke, Ph.D.
Professor, Director
Virginia Seafood Agricultural Research and Extension Center
102 South King Street
Hampton, Virginia 23669 USA[117,100,101,46,116,118,64,101,107,99,110,104,97,106,109]
-
Helen Crocker
Research Microbiologist
Virginia Seafood Agricultural Research and Extension Center
102 South King Street
Hampton, Virginia 23669 USA
Tagged With
Related Posts

Aquafeeds
A look at protease enzymes in crustacean nutrition
Food digestion involves digestive enzymes to break down polymeric macromolecules and facilitate nutrient absorption. Enzyme supplementation in aquafeeds is a major alternative to improve feed quality and nutrient digestibility, gut health, compensate digestive enzymes when needed, and may also improve immune responses.

Health & Welfare
Aiding gut health with a natural growth promotor
A study with Nile tilapia conducted in commercial production cages in Brazil showed the potential – in the absence of major disease threats – of a commercial, natural growth promotor that modulates the microbiota (inhibiting growth of pathogenic bacteria and promoting growth of beneficial bacteria) and inhibits quorum sensing.

Aquafeeds
A look at the SME controlled extrusion process
A study was conducted using a Twin-Screw Extruder equipped with Specific Mechanical Energy (SME) and Density Control valves, to determine the effect of SME on the water stability of shrimp feeds. Further research is needed to evaluate the performance.

Innovation & Investment
American Unagi brings eel farming back ‘home’
Sara Rademaker launched American Unagi to shift eel farming to American soil, where the eels are from. Why? Because of the novelty, and because she saw an opportunity to do things better.


