System effectively removed solids and toxic nitrogenous wastes

Recirculating aquaculture systems (RAS), have been used to conserve water and manage water quality in aquaculture for several years. These systems remove fecal waste and toxic metabolites such as ammonia and nitrogen, kill pathogens that accumulate in the culture water, and reuse the same water over and over.
Most experimental and commercial recirculating systems are used in fish grow-out, and the technical feasibility of rearing fish larvae in RAS facilities has also been demonstrated. While RAS systems have been tested in shrimp hatcheries forbroodstock maturation, successful commercial rearing of shrimp nauplii to postlarvae has not been extensively reported.
The major challenge in raising shrimp larvae in recirculating systems is the tiny size of the larvae and even tinier size of the live organisms fed to the larvae. When water is removed from larval-rearing tanks, treated, and reintroduced to the tanks, the larvae and their food organisms should not be physically disrupted or removed in the process.
RAS system design
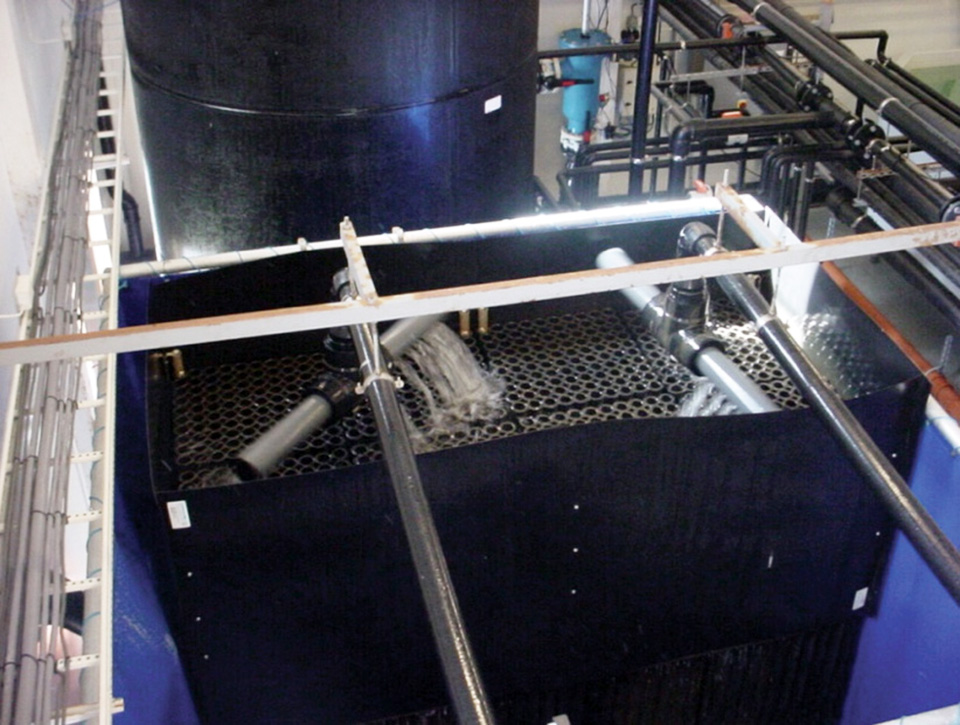
The author set up and operated an RAS system at Tanna Hatcheries, near Chennai, Tamilnadu, India, from November to December 2004. Fig. 1 shows a schematic outline of the system.
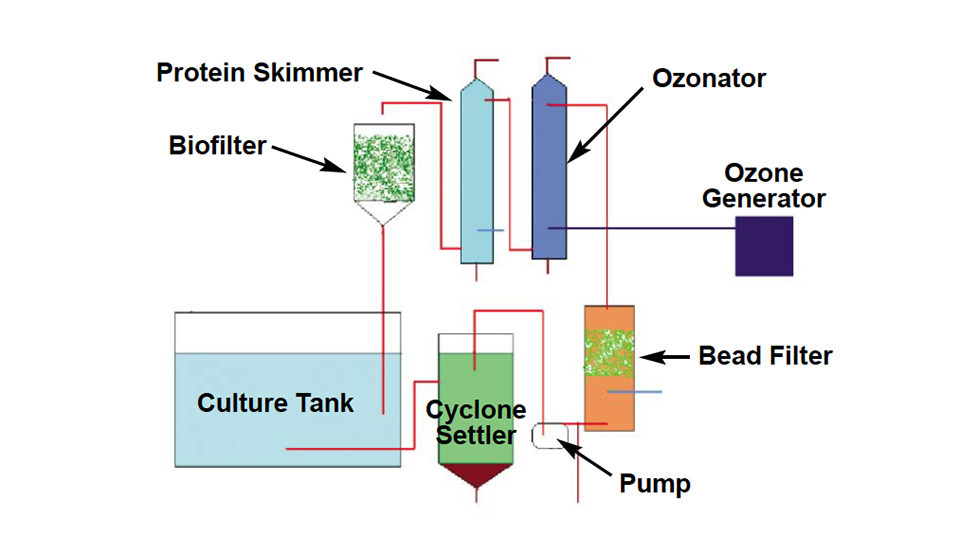
The system used a 1-cubic-meter fiberglass culture tank with a microscreen strainer on the tank bottom connected to a flexible hose to facilitate cleaning of the screen. The screen could be changed to use larger mesh sizes as the larvae grew. Water from the strainer entered a cyclone settler where heavier settleable solids were removed. The supernatant water from the settler was pumped through a bead filter for removal of suspended particles and some ammonia and nitrite.
After bead filtration, the water passed through a protein skimmer that used ozone to flocculate larger particles and inactivate pathogens. The water then went through a deozonator, where more dissolved solids were removed through foam fractionation and settling. The deozonated sea water entered a trickle-down biological filter for removal of most of the remaining ammonia and nitrite.
The 2,000-liter prototype system used 1,000 liters of water during larval rearing and its full capacity for post larvae. The RAS was initially cleaned and operated for 10 days prior to the start of larval culture. During this period, the system was periodically seeded with ammonium chloride to establish bacterial communities that detoxified nitrogenous waste in the biological filters.
Larval culture
Black tiger shrimp (Penaeus monodon) nauplii were stocked at the rate of 300 per liter in the culture tank. Once the nauplii metamorphosed into zoea, feeding with microalgae diatoms commenced. After the zoea metamorphosed to the mysis stage, a commercial, supplementary liquid feed was added along with the diatoms.
As the larvae became postlarvae, the density was reduced to 100 per liter. The postlarvae were fed artemia nauplii in addition to the liquid feed. Although routine commercial antifungal, ethylenediaminetetraacetic acid, probiotic, and vitamin C hatchery treatments were used, no antibiotics were applied during the 29-day rearing period. The postlarvae were routinely inspected and found healthy.
The survival in the larval phase was about 66 percent. Survival for the postlarvae was about 86 percent (Table 1). Although they took extra time to reach postlarval stages – probably due to low temperatures and high density – all animals were of a uniform size and had no attachments, swollen guts, or necrosis.
Ghanekar, Density and survival of shrimp larvae, Table 1
| Stage | Stocking Density | Harvest Density | Survival Rate |
|---|---|---|---|
| Nauplii to PL4 | 300/L | 198/L | 66.0% |
| PL4 to PL15 | 99/L | 85/L | 85.8% |
Water quality
Water salinity was 29 ppt throughout the trial, and temperature varied 26 to 27 degrees-C. The rate of daily recirculation was increased from an initial 600 percent to 1,400 percent at the end of the trial. About 5 to 20 percent make-up water was used every day. Water quality parameters were stable and within the limits recommended for shrimp larval culture. During the entire rearing period, the water transparency was high compared to normal hatchery tanks, and the animals were very active.
Results
The prototype RAS functioned effectively to remove solids and toxic nitrogenous wastes in the system, as well as kill microorganisms. The system could potentially be further improved for large-scale hatchery use, as well as grow-out and broodstock development applications.
While the initial capital cost of filters, ozone generator, and other equipment in the RAS were high, the cost savings associated with their use should also be considered. RAS systems require smaller areas than other systems because they support higher densities, and hence reduce construction costs and feed waste. Compared to conventional hatchery systems, the RAS approach requires much less water and lowers costs associated with pumping, disinfecting, and storing culture water.
In the present trial, feed use was lowered 30 to 40 percent. All other operating costs were also substantially reduced due to the small volume of water. The stable water quality in the rearing tank reduced stress to the animals and hence reduced infections. Fecal matter and uneaten feeds were constantly removed from the tank water, so there were less substrate and nutrients for sudden blooms of bacteria.
Enhanced biosecurity
The greatest advantage of rearing shrimp in this type of recirculating system was the reduction in the risk of diseases caused by pathogenic microorganisms. The culture water was continuously treated by ozone that inactivated most microorganisms. Since new water use was limited, the chances of new pathogens entering the system were minimized.
(Editor’s Note: This article was originally published in the June 2005 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Anil Ghanekar, M.Sc.
Aquaculture Consultant
Shakti Niwasam, 16/1 K.K. Road
Valmikinagar, Thiruvanmiyur
Chennai 600041 Tamilnadu, India[109,111,99,46,111,111,104,97,121,64,114,97,107,101,110,97,104,103,108,105,110,97]
Tagged With
Related Posts

Responsibility
A look at unit processes in RAS systems
The ability to maintain adequate oxygen levels can be a limiting factor in carrying capacities for RAS. The amount of oxygen required is largely dictated by the feed rate and length of time waste solids remain within the systems.
Innovation & Investment
A review of unit processes in RAS systems
Since un-ionized ammonia-nitrogen and nitrite-nitrogen are toxic to most finfish, controlling their concentrations in culture tanks is a primary objective in the design of recirculating aquaculture systems.

Intelligence
4-hexylresorcinol: sulfite-free control for melanosis in crustaceans
4-hexylresorcinol in a nonsulfite processing treatment against melanosis in crustaceans inhibits natural enzymes for shell hardening.

Health & Welfare
A case for better shrimp nutrition
Shrimp farm performance can often be below realistic production standards. Use proven nutrition, feeds and feeding techniques to improve profitability.


