Concept increases average pond survival rates and yields in WSSV-infected areas

The use of intensive nursery systems for the production of juvenile shrimp under biosecure conditions is an important WSSV management tool. Preliminary production results from several WSSV-infected shrimp farms in Ecuador suggest that stocking juveniles in grow-out ponds significantly improves shrimp survival and yields.
Here we describe the use of raceway systems for the production of juvenile Pacific white shrimp (Litopenaeus vannamei) in Ecuador. Most data presented are based on over 40 intensive nursery production cycles at the Semacua maturation/hatchery complex in Salinas, Ecuador. Supplemental data is also included from several nursery trials and pond productions at the Pesquera Industrial Bravito S.A. shrimp farm near Machala, Ecuador.
Semacua complex raceway system
This facility is currently producing a stronger WSSV-free juvenile shrimp to stock about 3,000 ha of grow-out ponds with three production cycles targeted per year. This system also has the potential to shorten the production cycle in the grow-out ponds.
The system has 12 concrete, 120-cubic-meter (30 x 3.35 x 1.2 m) HDPE-lined raceways enclosed in greenhouses. Aeration is provided by two regenerative air blowers (total capacity 25 hp). Each raceway is equipped with seawater and freshwater intakes, a 10.2-cm outlet, six banks of four 7.6-cm airlift pumps, six 0.9-meter-long air diffusers, a center partition, a bottom spray bar manifold, a Venturi injector with check valve, a 3-hp high-head pump, and a 83-cm rapid sand filter (0.56 square meters – 454 lpm).
System management

Management of the system includes water chlorination, algal inoculation, water quality and feed monitoring and PL health and growth monitoring. Seawater from the facility well point is used to fill the raceways. Culture water is treated with 10 to 15 ppm chlorine followed by dechlorination after 24 hours using sodium thiosulphate. Raceways are maintained with no water exchange throughout the nursery period. Limited (chlorinated) makeup water is used to replace water lost to evaporation and backwashes of the sand filter.
Twenty-four hours before stocking, water is fertilized (NaNO3, NaH2PO4, Na2SiO3 and Sequestrine at concentrations of 2.0, 0.2, 0.5 and 1.0 ppm, respectively) and inoculated with marine diatoms (Chaetoceros muelleri or C. gracilis) at an initial algal density of 4.0×104 cells per milliliter. Newly hatched artemia nauplii and commercial feeds (Zeigler PL Ready Reserve and Rangen #0 crumble) are added to the raceway prior to PL stocking.
Stocking and feeding
Raceways are stocked with PL7-15 at densities ranging between 20 and 80 PL per liter. All PL are tested for WSSV infection two to three days prior to stocking using one- or two-step PCR tests. WSSV-positive and WSSV-negative animals are stocked into different raceways. Commercial feeds are offered every four hours. Daily rations are reduced gradually from 25 percent of the total biomass to 12.5 percent before harvest.
Growth samples are checked every three days and feed is adjusted as needed. Dissolved oxygen, pH, temperature, salinity, and PL health are checked daily, while TAN, NO2, and NO3 are checked every three to five days. Nursery duration varied in most cases between 14- and 16-day cycles. Shrimp survival at harvest was estimated using both volumetric and weight methods.
Bravito production facility
Several nursery productions at the Bravito facility were conducted in nursery facilities and in six 50-cubic-meter (15×3.5×1.12 meters) HDPE-lined, greenhouse- enclosed raceways. Unlike the water management used at Semacua, raceways were maintained with 40 percent daily water exchange using untreated water passed through a series of filters going down in mesh size from 500 to 100 microns in a 25-cubic-meter sedimentation chamber.
Shrimp were fed artemia nauplii enriched with Aquagrow DHA, astaxanthin, and cod liver oil from 18 to 24 hours after hatching and Zeigler PL Ready Reserve No. 0 and No. 1. Raceways were stocked with both WSSV-free and WSSV-infected PL at 30 to 50 PL per liter. Nursery duration varied between 12 and 30 days.
Water quality
During the first week after stocking, only minimal water circulation was needed to maintain adequate water quality. Daily water usage was reduced from 300 percent to less than 1 percent per day. Raceway water filtration and circulation were initiated after seven to eight days. Throughout the nursery trials there was no need for medicated feed to control disease outbreaks.
Although ammonia and nitrite levels in the raceways were occasionally observed within ranges reported to be lethal to juvenile shrimp, shrimp growth and survival in all but one case was high.
Survival and growth
Survival rates averaged 69 percent (volumetric estimation method) and 80.5 percent (weight technique). The weight technique was found to be more accurate than the volumetric method. Although stocking density negatively affected the mean weight of the PL at harvest, PL growth stayed exponential for the stocking densities tested as PL growth rates doubled approximately every three to four days. Furthermore, after 10 to 12 days, PL growth increased drastically. This same increase in growth after about 12 days was also noticed at the Bravito farm.
Stocking densities greater than 70 per liter were found to debilitate the raceway system, causing PL to become weak and, in some cases, reflect increased mortality.
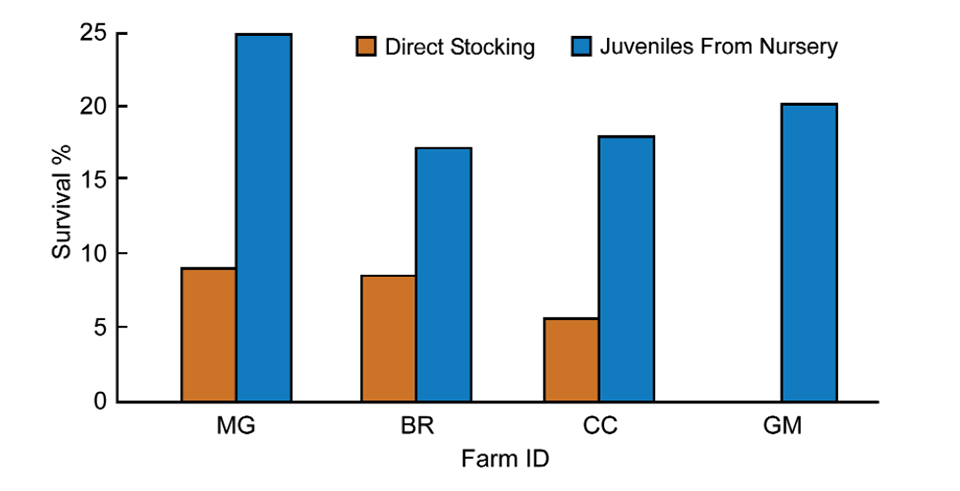
Ponds stocked with juveniles from these raceways rather than PL10 have shown improvement of up to 16 percent increase in survival (Fig. 1). Table 1 summarizes the data obtained from Bravito’s farm for ponds stocked with juveniles coming from nursery facilities, while Table 2 shows the effect of direct stocking on pond performance.
Samocha, Results from Bravito’s farm in Ecuador show the beneficial effect of stocking grow-out ponds, Table 1
| Pond Size (ha) | Stocking (PL/m2) | Cycle (days) | Survival (%) | Average Harvest Weight (g) | FCR | Yield (kg/ha) |
|---|---|---|---|---|---|---|
| 13 | 6.6 | 125 | 44 | 13.7 | 0.9 | 408 |
| 13 | 6.6 | 126 | 40 | 12.7 | 1.2 | 343 |
| 9 | 6.6 | 121 | 39 | 14.3 | 1.1 | 377 |
| 9 | 6.6 | 122 | 51 | 11.5 | 1.0 | 400 |
| 10 | 6.6 | 122 | 42 | 14.9 | 1.0 | 421 |
| 10 | 6.6 | 124 | 44 | 15.5 | 0.8 | 462 |
Samocha, Results from Bravito’s farm in Ecuador show the effect of direct stocking, Table 1
| Pond Size (ha) | Stocking (PL/m2) | Cycle (days) | Survival (%) | Average Harvest Weight (g) | FCR | Yield (kg/ha) |
|---|---|---|---|---|---|---|
| 11 | 5.8 | 141 | 28 | 13.4 | 0.7 | 220 |
| 13 | 6.1 | 139 | 27 | 12.9 | 0.6 | 216 |
| 10 | 8.6 | 130 | 40 | 11.2 | 0.5 | 352 |
| 11 | 8.1 | 123 | 22 | 15.5 | 0.6 | 284 |
| 13 | 7.3 | 133 | 21 | 14.7 | 1.0 | 228 |
| 3 | 7.6 | 140 | 28 | 13.0 | 1.4 | 283 |
Conclusion
Stocking grow-out ponds with juvenile shrimp from raceways or other nursery facilities has increased average pond survival rates and yields in WSSV-infected areas of Ecuador. Pond survival rates with raceway-produced juveniles averaged 21 percent in the cold season (WSSV virulence is heavier during this season), and are expected to be higher for the upcoming warm season.
Survival rates should increase further when raceway technology is accompanied by other WSSV management procedures (i.e., genetic selection, biosecurity, etc.). Ammonia and nitrite buildup in the raceways can be avoided when stocking densities are low (less than 10 PL per liter), but when the raceways are stocked at high densities (greater than 40 PL per liter), diatom blooms are difficult to control and ammonia/ nitrite levels can become unmanageable. Additional work is currently underway to achieve stable diatom blooms and better water quality conditions with high stocking densities in the raceways.
(Editor’s Note: This article was originally published in the December 2000 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Tzachi Samocha, Ph.D.
Shrimp Mariculture Research Facility
Corpus Christi, Texas, USA[117,100,101,46,99,99,117,109,97,116,46,110,111,99,108,97,102,64,97,104,99,111,109,97,115]
-
Jorge Cordova
Farm Owner
Belere S.A.
Machala, Ecuador[99,101,46,111,114,112,46,111,101,64,97,118,111,100,114,111,99]
-
Todd Blacher
Special Project Manager
Empagran
Guayaquil, Ecuador[109,111,99,46,108,105,97,109,116,111,104,64,114,101,104,99,97,108,98,100,100,111,116]
-
Alex de Wind
Production Manager
Pesquera Industrial Bravito S.A.
Machala, Ecuador[99,101,46,116,101,110,46,97,117,99,101,64,100,110,105,119,101,100,97]
Tagged With
Related Posts

Intelligence
Changing paradigms in shrimp farming, part 2
The focus at Belize Aquaculture Ltd. was to use clean animals that perform well at high densities and in waters with high nutrient loads.

Health & Welfare
Biofloc technology: Possible prevention for shrimp diseases
Facing emerging viral problems and rising energy costs, the use of biofloc technology in biosecure systems offers an answer for sustainable shrimp aquaculture. The main attributes of biofloc systems in reducing disease risk include the fact that low water exchange improves pathogen exclusion.

Health & Welfare
Greenhouse systems a promising technique against WSSV in Ecuador
CENAIM’s greenhouse production trials were carried out at the commercial Pesglasa shrimp farm, located in the Taura region of Ecuador’s Guayas Province.

Health & Welfare
Mineral extract reduces EMS, WSSV impacts in Pacific white shrimp
The authors recently performed a study to evaluate immunity to white spot syndrome virus and virulent Vibrio parahaemolyticus in shrimp treated with a new mineral extract additive in pelletized feeds.


