Safety & efficacy
Further advances in aquatic bio-technology and aquaculture will likely be necessary to meet the growing world demand for freshwater and marine fish products. In general, there is an intense effort by many developing and industrialized countries to come up with technologies to improve production levels by enhancing reproduction, disease resistance, survival, feeding efficiency, and growth rates. The enhancement of growth rate is a particularly important economic parameter, as it can significantly reduce the time required to produce market-size fish.
Growth rate stimulation
Current methods to stimulate growth rate involve molecular, environmental, and pharmacological approaches. Investigators have primarily used two of these approaches to enhance growth rate in cultured fish, including the production of transgenic fish species that overexpress growth hormones and treatment of fish with exogenous recombinant growth hormones.
Results obtained by a number of investigators have demonstrated that the administration of exogenous recombinant growth hormone as a food supplement could be a viable method to enhance growth rates and feed conversion in cultured fish. However, the production and use of hormones can be costly. Thus, the development of methods for the efficient production of recombinant growth hormone has been a key step.
Biologically active recombinant growth hormone has been successfully produced in Escherichia coli, Bacillus subtilis, Pichia pastoris yeast, and plants such as canola.
Concerns
However, there is concern over the safety of hormone-fed fish destined for human consumption. In this context, the issue of food safety of transgenic animals has been addressed internationally by the Food and Agricultural Organization, World Health Organization, and Organization for Economic Cooperation and Development, which also convened a special symposium on aquatic bio-technology.
These groups concluded that bio-technology and other modern techniques do not inherently result in foods that are unsafe, and the safety of foods produced using recombinant techniques should be further investigated on a case-by-case basis.
The safety of fish fed recombinant growth hormone is clearly a different situation from the production of genetically modified animals. One of the key advantages of the feeding approach is that the cultured fish are not genetically modified and therefore do not car-ry transgenes and vectors, which has been of concern to interested groups. Nor is there a concern over the spread of genetically modified animals, since hormone-fed animals are not different from wild species.
BST analogy
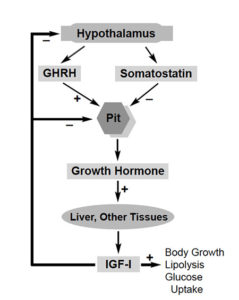
A more appropriate analogy, with a few notable differences, is the use of bovine growth hormone in cows. The U.S. Food and Drug Administration (FDA) approved the use of recombinant bovine growth hormone or somatotropin (BST) for increasing milk production in dairy cows almost a decade ago. BST increases milk output by supplementing cows’ natural growth hormone, which is produced in the pituitary gland.
Milk from treated cows has the same nutritional value and composition as milk from untreated cows. In fact, as indicated by an FDA commissioner, BST “has been one of the most extensively studied animal drug products to be reviewed by the agency.” He concluded that the public can be confident that milk and meat from BST-treated cows is safe to consume.
In this context, more than 120 studies examined the human safety of recombinant BST, as summarized in the August 24, 1990, issue of Science magazine. However, independent scientists criticized FDA for ignoring scientific evidence concerning the consumption of insulin-like growth factor-I (IGF-I), which increases in milk following treatment with BST. IGF-I is a natural growth factor that is produced in liver and other tissues following increased levels of growth hormone.
While BST structure in humans is sufficiently different from cows, the IGF-I structure in cows is very similar to the IGF-I in humans. IGF-I normally regulates cellular growth response. The concern is that overproduction of IGF-I in milk may cause inappropriate cell division in humans who consume milk from BST-treated cows, leading to accelerated tumor growth.
Safeguards
The situation is very different in nonmammalian vertebrates. Fish growth hormones and IGF-I molecules are structurally different from those of humans. In this regard, fish are among the most variable species in vertebrates, and as such, significant variations exist in the structure of peptide hormones and their receptors. For example, the carp growth hormone used in the author’s studies to enhance fish growth rate in cyprinid and salmonid fish has only a 35 percent sequence similarity compared to human growth hormone.
While complete information on the sequence differences between growth hormone receptors in fish and humans is not available, it is generally accepted that human growth hormone receptors do not respond to hormones from other species. This is one of the bases for FDA approval of BST for increasing milk production in dairy cows, as bovine growth hormone has no significant activity in humans.
Carp growth hormone will likely have no effect on humans. Furthermore, there is greater variation between fish IGF-I molecules and human IGF-I, which reduces the risk that fish IGF-I might have activity in humans.
A further natural safeguard for the consumption of fish treated with growth hormone is that unlike fish intestines, adult human guts lack the ability to absorb intact proteins such as growth hormone.
Low levels
An additional safeguard is that in fish, growth hormone-induced IGF-I production is significantly lower than in mammals. Studies by the author that appeared in the volume III, 1998, issue of General and Comparative Endocrinology found it difficult to detect IGF-I in goldfish by standard protein immunoassay following treatment with recombinant carp growth hormone. Researchers had to detect the IGF-I by reverse-transcriptase polymerase chain reaction, which is a very sensitive molecular technique.
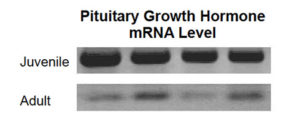
It should be noted that the hormone dose required to induce growth in fish is relatively small. The author’s trials induced significant growth enhancement following feeding of recombinant carp growth hormone at a rate of 200 µg per kilograms of fish body weight. The final concentration of growth hormone following absorption in the blood would be comparable to that normally detectable in fast-growing juvenile fish, which have significantly higher hormone production than adult fish.
Fast clearance
In a study published in the volume 28, 2003, issue of Fish Physiology and Biochemistry, the author investigated the gastrointestinal uptake of recombinant growth hormone in rainbow trout. The results demonstrated that orally administered recombinant growth hormone absorbs rapidly through the fish gastrointestinal tract and disappears after a short time from the system.
No trace of the orally administered growth hormone was found in the blood, gut, muscles, or liver after 90 minutes of administration. Therefore, fish farmers could easily stop feeding a diet containing growth hormone a few days prior to marketing to assure fish free of recombinant hormone residues.
Conclusion
There is overwhelming evidence, both from environmental and human health viewpoints, in support of the oral administration of recombinant fish growth hormones as a safe method of enhancing growth rates in cultured fish. Recombinant growth hormones can benefit the world’s growing aquaculture industry and provide a more acceptable approach to enhanced growth than the production of transgenic fish.
However, by the nature of the ongoing debate on genetically modified organisms in the marketplace, it is likely that all methods of enhancing growth rate in fish will be open to questions and criticisms from environmental groups. It is essential for the further development of oral recombinant fish growth hormones to address these concerns and provide information on the safety of the approach.
(Editor’s Note: This article was originally published in the August 2004 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Hamid R. Habibi, Ph.D.
Professor
University of Calgary
2500 University Drive N.W.
Calgary, Alberta, Canada T2N 1N4[97,99,46,121,114,97,103,108,97,99,117,64,105,98,105,98,97,104]
Related Posts

Health & Welfare
Antigens provide immunity against ich in channel catfish trials
Vaccination against the Ich parasite is an alternative to chemical treatment. Fish develop a humoral immune response to trophont antigens, with the degree of protection related to the immunizing doses of trophonts used.
Health & Welfare
Developing live bacterial vaccines by selecting resistance to antibacterials
As wide use of antibiotics has led to antibiotic resistance in fish pathogens, vaccines present an alternative control method to prevent bacterial diseases.

Health & Welfare
Fish vaccines in aquaculture
Vaccines are a proven, cost-effective method to prevent infectious diseases in animals. Current vaccinations for fish can be categorized as killed fish vaccines or modified live vaccines.

Innovation & Investment
Microalgae into medicine: Biotech startup targets shrimp, salmon diseases
MicroSynbiotiX is employing the power of transgenic microalgae to make it cheaper and easier for aquaculture producers to administer vaccines to fish.


