Determining amounts of major cations needed to counteract ionic imbalance challenging

Low concentrations of potassium, and occasionally of magnesium, may adversely affect shrimp survival and growth in low-salinity water. It is common to apply mineral salts such as potassium chloride, potassium magnesium sulfate, magnesium sulfate, or magnesium chloride to low-salinity shrimp ponds (Table 1). However, determination of amounts of these salts needed to counteract ionic imbalance is problematic, because minimum concentrations of major cations (sodium, potassium, calcium, and magnesium) required for physiological functions in penaeid shrimp are not known with certainty.
Boyd, ionic imbalance, Table 1
| Name | Formula | Composition % Potassium | Composition % Magnesium |
|---|---|---|---|
| Muriate of potash | KCl | 50 | 0 |
| K-Mag (R) | K2SO4·2MgSO4 | 17.8 | 10.5 |
| Epsom salt | MgSO4·7H2O | – | 9.86 |
| Magnesium chloride | MgCl2·6H2O | – | 12.0 |
Ionic imbalances
I became aware of ionic imbalance in shrimp years ago, because a farmer in Ecuador sent me a water analysis report for a low-salinity pond (8 ppt) in which postlarvae would not live. Concentrations of calcium, magnesium and sodium were normal (around 100 mg/L, 425 mg/L, and 2,500 mg/L, respectively), but potassium concentration was below 1 mg/L. The normal potassium concentration for seawater diluted to 8 ppt would have been around 90 mg/L.
Although I recommended adding only 50 mg/L potassium, after potassium addition, shrimp postlarvae survived well in the water. The next year, a new inland shrimp farm with pond water salinities of around 2 ppt was established in Alabama. During the first month of culture, heavy shrimp mortality occurred. Water analyses revealed around 6 mg/L potassium. I suggested raising potassium concentration to 50 mg/L, because this rate had been successful in Ecuador. Shrimp mortality ceased following the potassium addition.
In normal seawater, the ratio of major ions (sodium:magnesium:calcium:potassium) is 27:3:1:1 (Table 1). The seawater equivalent concentrations of major cations in low-salinity water can be estimated from the ratio of each major cation (in milligrams per liter) to the salinity of normal seawater (Table 2).
To achieve the seawater equivalent concentrations of potassium and magnesium in a pond, the factors 11.01 and 39.1, respectively (Table 2), should be multiplied by salinity. For example, at a salinity of 2 ppt, seawater equivalent concentrations would be 22 mg/L potassium and 78 mg/L magnesium.
Boyd, ionic imbalance, Table 2
| Concentration in normal seawater* (mg/L) | Factor | |
|---|---|---|
| Sodium | 10,500 | 304.35 |
| Magnesium | 1,350 | 39.13 |
| Calcium | 400 | 11.59 |
| Potassium | 380 | 11.01 |
*34.5 ppt salinity.
Ionic concentrations in low-salinity waters do not result simply from dilution of seawater. Their concentrations depend on types, amounts, and solubilities of minerals in soils and other geological formations with which the pond water had contact and upon climate. Proportions of major cations in saline surface and groundwater may differ greatly than those in the ocean and estuaries.
Sodium and potassium both bear a single negative charge, and shrimp uptake of potassium may be influenced by the concentration of sodium and vise-versa. The same logic applies to magnesium and calcium uptake by shrimp. Moreover, shrimp absorb ions across their gills on a molar concentration basis rather than a weight-volume concentration basis. The molar concentration of a major cation is its concentration in grams per liter divided by its atomic weights (sodium, 23 grams; potassium, 39.1 grams; calcium, 40.08 grams; magnesium, 24.31 grams). The molar concentration ratio of the four ions in normal seawater is roughly 46:6:1:1.
Treatment rates and molar ratios
It has been suggested that molar ratios of sodium:potassium and calcium:magnesium found in seawater might be ideal in low-salinity shrimp ponds. These ratios are 47.1 and 0.018, respectively. Treatment rates for potassium and magnesium can be made on a molar basis as follows:
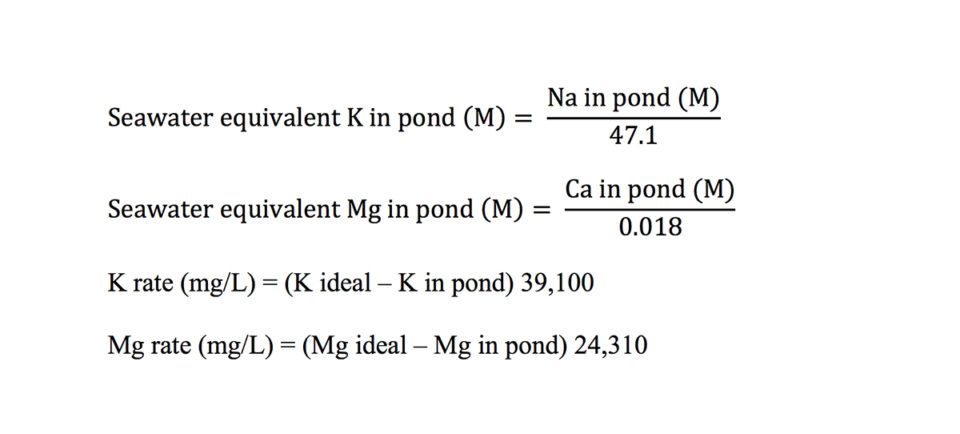
There often is little difference in treatment rates calculated as molar concentrations or weight/volume concentrations. For example, suppose a water of 5 ppt salinity contains 1,800 mg/L (0.0783 M) sodium, 25 mg/L (0.000639 M) potassium, 60 mg/L (0.0015 M) calcium, and 20 mg/L (0.000823 Mg magnesium. The potassium and magnesium treatment rates for seawater equivalent concentrations are 30 mg/L and 176 mg/L, respectively, while the respective treatment rates for seawater equivalent ratios (Na/K and Ca/Mg) are 40 mg/L and 182 mg/L.
Greater treatment rates prudent
Seawater equivalent proportions of cations calculated above represent higher concentrations than necessary for shrimp, because shrimp can maintain normal physiological function over a rather wide range of ionic concentrations. However, use of the greater treatment rate is prudent.
Concentrations of potassium and magnesium steadily decline following treatment because of uptake on the two elements by cation exchange sites in bottom soil and by fixation of potassium within the interlayers of clays. Rainfall dilutes ionic concentrations and ions are lost in overflow. Ponds must usually be treated two or three times during a 100- to 160-day grow-out period. In Alabama, ponds that have been treated annually with potassium and magnesium salts for 16 consecutive years still require treatment.
Potassium concentration usually is more critical than the magnesium concentration, and I doubt that the benefits of magnesium application are usually cost effective. I suggest initially trying only potassium treatment. The minimum concentration of potassium is unknown, and it no doubt varies with salinity and concentrations of other ions. A treatment rate of 20 to 30 mg/L is probably adequate, but to extend the time until re-treatment is necessary, 50 to 75 mg/L potassium can be applied. The potassium concentration should be monitored at monthly intervals to assure adequacy.
In addition to having physiologically acceptable concentrations of major cations, aquaculture pond waters should have bicarbonate concentration above 60 mg/L (»50 mg/L total alkalinity as CaCO3) and calcium hardness concentrations at least as great as total alkalinity (50 mg/L calcium hardness = 20 mg/L calcium).
Alkalinity is necessary to provide inorganic carbon for aquatic plants, and alkalinity and calcium buffer the water against pH rise resulting from the removal of carbon dioxide and bicarbonate from water for use in photosynthesis. Agricultural lime is used to increase alkalinity and hardness concentrations, but the calcium concentration and calcium hardness also can be increased by calcium sulfate application. It requires 1.72 mg/L of calcium sulfate (CaSO4·2H2O) to provide 1 mg/L of calcium hardness (0.4 mg/L of calcium).
Author
-

Claude E. Boyd, Ph.D.
Professor Emeritus
School of Fisheries, Aquaculture and Aquatic Sciences
Auburn University
Auburn, Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Responsibility
Electrical conductivity of water, part 1
The electrical conductivity of water, usually called specific conductance or simply conductivity, is an important property of water frequently measured in aquaculture systems, and provides an assessment of the total concentration of dissolved ions in water.

Health & Welfare
The importance of iron in aquaculture systems
Iron is the fourth most abundant element in the earth’s crust, but occurs at very low concentrations in surface waters and oceans. It is an essential element for many organisms as part of many enzymes, and also has an important role in plant photosynthesis.

Responsibility
Inorganic fertilization of production ponds
Nitrogen and phosphorus are the most important nutrients in fertilization of both freshwater and coastal ponds. Many factors that can affect the response of ponds to fertilizers are location-specific. Aquaculture pond managers will have to figure out the best procedure for a given location or even for an individual pond.

Responsibility
Reviewing the ‘near-magical’ properties of water
Understanding the hydrologic cycle, water’s unique physical properties and their behavior in aquaculture systems – phases, humidity, saturation, stratification, light reflection and refraction – is pertinent to properly manage water quality in production systems, according to world-renowned expert Dr. Claude Boyd.

