Bacterial cultures modify microbial communities, reduce pathogens and improve growth, survival
Most marine hatcheries encounter bacterial problems that negatively affect production. Live feed, which is still essential in the larval rearing of fish and shrimp, is also a significant source of bacterial contamination in larval-rearing tanks. For example, the concentration of bacteria in hatching tanks for artemia can reach 107 bacteria per milliliters water after 24 hours of incubation, with a high proportion of potentially hazardous Vibrio sp.
For this reason, hatcheries apply water treatments such as ultraviolet irradiation, ozonation, membrane filtration and the addition of antibiotics and disinfectants to reduce bacterial loads in the culture water. However, these approaches disturb the balance of microbial communities and favor uncontrolled growth of opportunistic and potentially pathogenic bacteria. Furthermore, the prophylactic use of antibiotics in aquaculture may lead to the selection of bacteria that are drug-resistant or more virulent, and should be avoided.
Probiotics offer a treatment alternative. These bacterial cultures modify the microbial communities in water and sediment, reduce or eliminate pathogenic microorganisms, and generally improve the growth and survival of the targeted species.
Probiotic development
The development of suitable probiotics requires basic and applied fundamental research, full commercial-scale trials, and appropriate monitoring tools. As part of a European Union-funded research project, the authors investigated the potential of using selected bacterial strains for preemptive colonization of the culture environment for specific stages of larvae.
Selection steps
The selection procedure included the following steps:
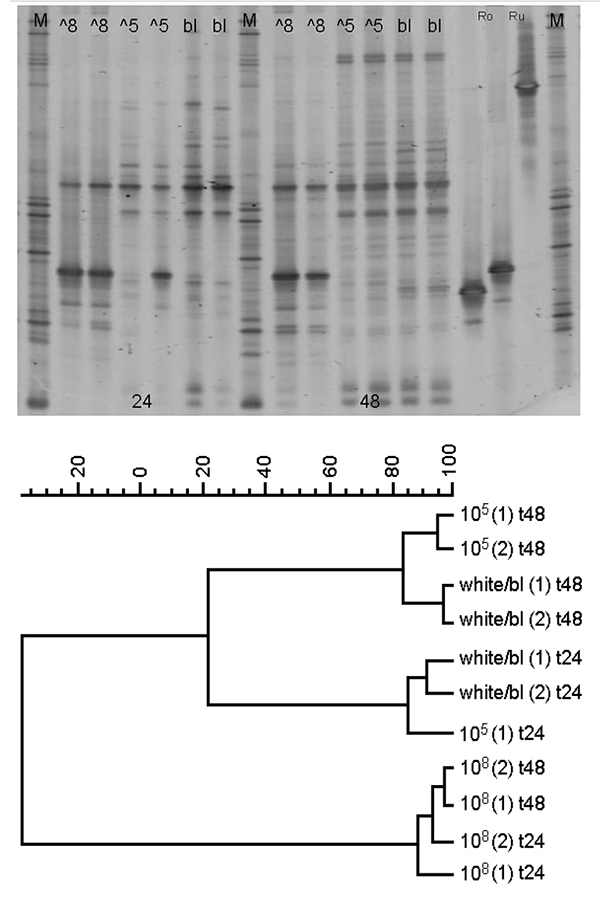
- The strains or mixtures of bacteria were screened in vivo with a focus on the ability of the probiotics to improve the performance of live food production or diminish the risk of transmitting pathogens to the fish larvae. This was accomplished by reducing the concentration of vibrios in the rearing water or colonizing the artemia per rotifers. In the latter case, the dose of probiotics applied to the rearing system to influence the bacterial community must be assessed (Fig. 1).
- Optimal growth factors of the bacteria were considered in relation to the prevailing environmental conditions such as temperature, pH, and salinity in the aquaculture production systems in which the bacteria would be utilized.
- The authors carried out a first estimation of the pathogenicity of the best-performing strains based on their molecular identification as well as published information. As a consequence, Vibrio strains and bacteria classified by the U.S. National Institutes of Health as “risk group 2 microorganisms,” agents associated with human disease that is rarely serious and for which preventive or therapeutic interventions are often available, were discarded.
- The resistance of these bacterial strains to current human and animal therapeutic antibiotics was investigated using plating methods with different antibiotic concentrations to exclude antibiotic-resistant bacteria from future applications. Their safety toward the target organisms was obviously evaluated during the initial screening, and their lack of toxicity toward other aquaculture organisms was also assessed. For example, a probiotic cocktail leading to higher rotifer production could include bacterial strains that might be opportunistic pathogens to sea bream under particular conditions.
- This was followed by field testing of the revised mixture of bacteria, taking into consideration that removing bacterial strains from a performing mixture might lead to poorer performance, since the functions of the individual strains were not always identified.
- The final stage was registration. Regulations for the incorporation of microorganisms in animal feed have changed recently, and applications for fish are still to be drafted. Microorganisms for rotifer and artemia culture might remain free from specific regulation.
Probiotic application
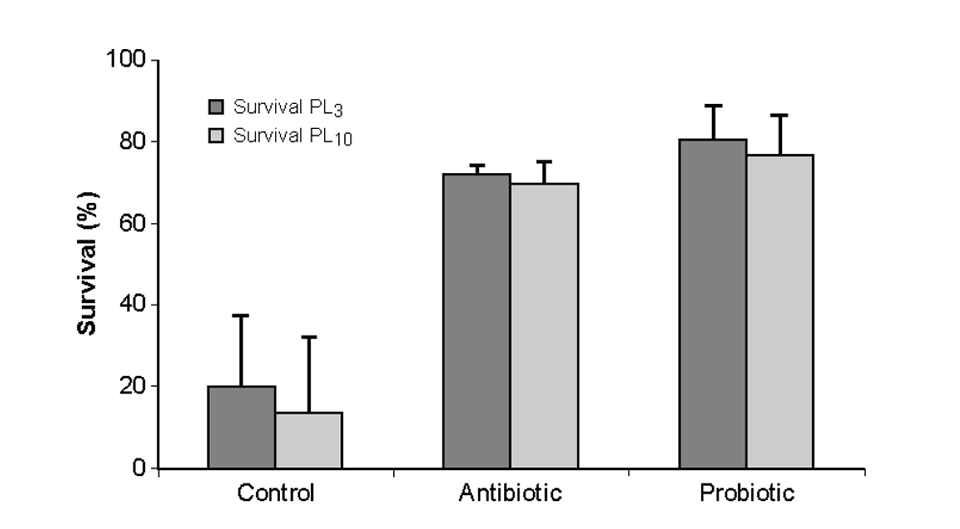
A similar screening method was applied for the development of INVE Sanolife PRO-1, a probiotic product for shrimp hatcheries. Daily applications of the mixture of bacteria led to a significant improvement in larval shrimp cultures in terms of survival rates and biomass production compared to the control treatments (Fig. 2).
Conclusion
Concerns with respect to the use of microorganisms in animal rearing are growing. This new era for probiotics – one of registration and control, transparency, safety and efficacy – is to the advantage of farmers, producers and consumers.
(Editor’s Note: This article was originally published in the August 2004 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
O. Decamp
INVE Technologies NV
Hoogveld 93 – 9200
Dendermonde, Belgium[101,98,46,101,118,110,105,64,112,109,97,99,101,100,46,111]
-
P. Makridis
CCMAR
University of Algarve
Gambelas, Faro, Portugal -
T. Vercauteren
Avecom NV
Belgium -
K. Dierckens
Artemia Reference Centre
Ghent University
Belgium -
K. Van Driessche
LabMET
Ghent University
Belgium
Related Posts

Health & Welfare
Artemia bioencapsulation delivers probiotics via digestive tracts of fish larvae
Research with sturgeon and carp species indicated that encapsulated artemia has high potential to carry probiotics or other beneficial microorganisms.

Health & Welfare
Assessment of supplemental Bacillus probiotics in whiteleg shrimp juveniles
Results of a feeding study supplementing probiotics in the diet showed that when the Bacillus species were complemented in an appropriate concentration into feeds, the growth and feed efficiency of whiteleg shrimp could be improved.

Health & Welfare
Bacillus probiotics benefit tilapia rearing under challenging conditions in Brazil
A recent study that evaluated the benefits of using probiotics with a balanced mixture of Bacillus bacteria strains to inhibit pathogenic bacteria in tilapia.

Health & Welfare
Bacillus probiotics improve hatchery, nursery production in EMS-hit Mexico
In early 2014, a trial to evaluate the effects of a mixture of Bacillus strains on early mortality syndrome bacteria during the larviculture and nursery phases for shrimp was carried out at a commercial hatchery in Mexico.


