Efficient oxygenation of saline water at up to 30 PPT

Bubble size is a critical parameter in diffused bubble, aquaculture aeration systems. A study conducted to determine the influence of water with different salinities on performance of nanobubble generating technology (NBT) showed that by using the same amount of power and air flow, the amount of oxygen transferred by NBT into water at a salinity of 30 ppt is 8.5 times that transferred into city (fresh) water.
Aeration in aquaculture
Natural oxygenation by diffusion from the air and photosynthesis by aquatic plants will not meet the combined oxygen demand of the cultured species and the other biota in intensive aquaculture. The efforts to increase dissolved oxygen (DO) concentrations in ponds include partial water exchange to replace the oxygen-deficient water with oxygenated water from the source, addition of fertilizer to stimulate photosynthesis by aquatic plants, applications of compounds such as hydrogen peroxide that release oxygen through chemical reactions, adding pure oxygen gas into pond waters, and use of mechanical aerators. Water exchange and aeration are the most widely used and effective techniques.
In semi-intensive and intensive aquaculture, aerators are necessary to satisfy the oxygen requirements for respiration of the cultured organisms. Sufficient aeration also is essential for ammonia management by promoting nitrification. Different types of aeration are used in aquaculture. Aeration is performed through two methods: splashing water into air with devices such as vertical pumps, pump sprayers and paddle wheels; or releasing bubbles of air into water with propeller-aspirator-pumps and diffused-air systems. There is increasing interest in fine-bubble diffusers as a means of aerating aquaculture system─ particularly in highly-intensive grow-out or holding units.
Micro-nanobubbles
Microbubbles (MBs) and nanobubbles (NBs) are tiny bubbles with a respective diameter of 10–50 µm and <200 nm, and have been explored for various applications. A micro-nanobubble (MNB) is a bubble that ranges in diameter from 0.1 micrometers to 10.0 micrometers. MNBs technology has attracted much interest in bio-related areas such as bioremediation, aquaculture and plant cultivation. MNBs are superior to macro-bubbles because they have larger interfacial area, higher inner pressure, lower rising velocity in water, long lifetime and high mass transfer efficiency. Also, the generation method of MNBs has an impact on their properties. All these factors will dramatically improve dissolved oxygen supply.
Study setup
It is common to report aerator performance parameters at standard conditions to overcome site specific conditions. The aerator performance tests are standard oxygen transfer rate (SOTR) (mass transferred per unit time) kg O2/hr; and standard aeration efficiency (SAE) (mass transferred per unit time per unit power) kg O2/kW-hr. The procedure for conducting the tests followed the protocol suggested by the American Society of Civil Engineers.
Surface aerators for use in aquaculture have been tested for oxygen-transfer characteristics in fresh water, but little is known about the influence of salinity on aerator performance. In order to determine the influence of water with different salinities on performance of nanobubble generating technology (NBT) (Blue Planet Environmental Inc.), we conducted a study at the Auburn University E. W. Shell Fisheries Center, Auburn, Alabama. Salinities of 5, 10, 20 and 30 part per thousand (ppt) were obtained by dissolving Crystal Sea® in city water (salinity = 0.1 ppt).
The procedure involved deoxygenating water in the experimental tank with sodium sulfite and cobalt chloride. Water was deoxygenated by adding 9.85 grams of sodium sulfite (Na2SO3) per cubic meter for each 1 part per million (ppm) of dissolved oxygen and 0.2 g cobalt chloride (CoC12. 6H20) per cubic meter of water. Cobalt chloride catalyzes the reaction of sodium sulfite with oxygen. Then, water was then re-oxygenated by NBT that was supplied with 0.7 l/min flow rate of air. During subsequent operation of NBT, dissolved oxygen concentrations were measured at 10 seconds intervals by using SMARTROLL™ RDO® Handheld oxygen meter (In-Situ Inc.) until DO concentration reached 80 % of saturation. For each appropriate salinity and temperature, DO concentration at saturation (Cs) was obtained.
For each DO measurement during an aeration test, the DO deficit was obtained by subtracting DO concentration in the tank from Cs. Oxygen transfer coefficient (KLaT) was calculated. Oxygen transfer is affected by water temperature. Thus, the value for the KLaT was adjusted to 20°C. The adjusted value (KLa20) was then used to calculate SOTR. The wire power was computed and divided into SOTR to provide the standard SAE. Oxygen-transfer tests were repeated for three times at each salinity.
Results
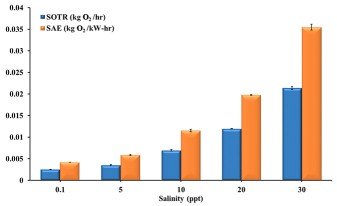
The SOTR and SAE of same NBT unit supplied with the same flow rate of air increased with increase in water salinity. NBT supplied with 0.7 l/min of air in water with 30 ppt had SAE 8.5 times greater than in city water (Figure 2). In other words, by using the same amount of power and air flow, the amount of oxygen transferred by NBT into water at salinity of 30 ppt is 8.5 times that would be transferred into city water.
Effect of bubble size
Bubble size is a critical parameter in diffused bubble systems because it determines the interfacial surface area, bubble-rise velocity, and mass-transfer coefficient. Oxygen transfer from air bubbles to water is related to bubble size. Smaller bubbles augment the oxygen transfer efficiency. If the same volume of air is introduced into water in two different spherical bubble diameters, the ratio of the total surface areas is inversely proportional to the ratio of the diameters. For example, at the same air flow rate, decreasing the bubble diameters from 2.5 mm to 0.5 mm would increase the interfacial contact area between the air and water by a factor of five for spherical bubbles. Thus, the fine bubbles will transfer oxygen to water more than that transferred by the coarse bubbles.
The goal in obtaining higher efficiencies for aeration or oxygenation of municipal water, wastewater or aquaculture tanks and ponds, is to reduce the gas flow and pressure so that less pumping energy is needed. Smaller bubbles have a larger surface area than the same volume of gas contained in larger bubbles. The greater total surface area of small bubbles means that the gas dissolves faster in the liquid which will result in reducing the required gas flow.
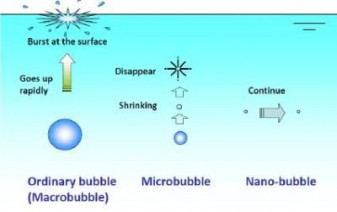
An important property of microbubbles that distinguishes them from the conventional large bubbles is that they shrink when their size is below a critical value. Ideally, bubbles should be of such a size that when they reach the surface they contain little or no air (or oxygen). At water depth of 3 m, a 5-mm bubble will lose only about 15 percent of its available oxygen, while a 2-mm bubble will lose about 50 percent. If the bubbles are too small, they will lose their oxygen very rapidly, and thereafter will have no valuable effect.
If the bubbles are too large, they will rise to the surface so quickly that there is insufficient time for oxygen transfer to take place (Figure 1). Since buoyancy decreases with bubble size, microbubbles float upward very slowly and have longer retention time in aqueous solutions. Small bubbles rise more slowly in the liquid than larger bubbles. This is because of an increase in the friction ratio of gas to liquid. This increase in friction with the liquid means that the gas bubbles can drag more liquid along as it rises therefore creating greater lift per volume of gas. Nanobubbles of nitrogen, methane, or argon with radius of 50 nm have a lifetime of more than two weeks. Some nanobubbles were proved to exist in water for months.

Mass transfer is the net movement of mass from one location to another. The decrease in bubble radius and the increase in bubble internal pressure increase the mass transfer efficiency from bubbles to surrounding liquid. The increased pressure results in the diffusion of entrapped gases from a high-pressure area inside the bubble to a lower-pressure environment of the surrounding aqueous solution, as described by Henry’s law. As a result, microbubbles will shrink further and finally collapse.
The rise velocities and mass-transfer coefficients of small bubbles are highly sensitive to bubble diameter. As a consequence of these properties, supplying oxygen in the form of microbubbles enables a high level of oxygen transfer applicable to oxygen consuming processes (e.g., aquaculture, hydroponic cultivation, aerobic fermentation, and aerobic treatment of sewage).
Effect of water salinity
The performance of different types of aerators respond differently to changes in water salinity. The aeration efficiency of vertical-pump aerators is not affected by water salinity. Salinities of 0 to 30 ppt have no obvious effects on oxygen transfer of Taiwan-style paddle wheel aerator either with two or four paddle wheels. However, diffused-air aeration systems such as propeller-aspirator-pump aerators are more efficient in brackish-water than in freshwater. Thus, this study was conducted to evaluate the effect of salinity on oxygenation by nanobubble devices.
The influence of salinity on aerator performance is a direct result of the influence of salinity on bubble size. Bubbles released by the propeller-aspirator-pump aerators were much smaller at salinities of 10 ppt and above than at lower salinities Nevertheless, above a salinity of 10 ppt, there was no enhancement in standard aeration efficiency (SAE) with increasing salinity. Moreover, performance of aerators is influenced by test tank geometry. Using small tanks in the freshwater tests results in lower SAE values. However, test tank geometry would not affect the influence of salinity on oxygen transfer, because bubble size is not related to tank geometry but to salinity.
For MNBs, salinity has no influence on their size, but does have a great impact on their performance. In different salinities, MNBs are negatively charged, which allows them to successfully adsorb positively charged microorganism. Moreover, when the bubble collapses, the ion concentration around the gas-water interface increases, resulting in radical generation, which may be related to the biological activity. The greater the dissolved oxygen enhancement, the shorter the bubble stagnation time is achieved at the salinity of 0.7 g/L compared to those at higher salinities.
Authors
-

Hisham A. Abdelrahman
Ph.D. candidate
The School of Fisheries, Aquaculture and Aquatic Sciences
Auburn University
Auburn, Alabama 36849-5419 USA[117,100,101,46,110,114,117,98,117,97,64,56,48,48,48,97,97,104]
-
Karen L. Veverica, Ph.D.
Director
E.W. Shell Fisheries Center
The School of Fisheries, Aquaculture and Aquatic Sciences
Auburn University
Auburn, AL 36849-5419 USA[117,100,101,46,110,114,117,98,117,97,64,108,107,114,101,118,101,118]
Related Posts

Intelligence
Indoor-raised shrimp find potential market in Kentucky test
By raising shrimp in a closed building, producers can increase biosecurity, produce shrimp more consistently, grow shrimp year-round and locate production centers near markets. Chefs and consumers were very accepting of whole fresh shrimp, offered at a farmers market, that was farmed indoors in Kentucky.

Health & Welfare
Phytoplankton a crucial component of aquaculture pond ecosystems
Phytoplanktonic organisms, or microalgae, are very abundant in aquaculture ponds and have critical roles in these ecosystems, significantly influencing overall pond ecology and water quality. Proper management of phytoplankton populations is essential for successful aquaculture pond production.

Health & Welfare
Measuring susceptibility differences to IHHNV infection
This study evaluated the susceptibility to IHHNV in three different shrimp batches by measuring infectivity titers. Each batch showed a different infectivity titer, therefore, each batch had a specific susceptibility to IHHNV.

Health & Welfare
Acclimating shrimp postlarvae before pond stocking
Shrimp postlarvae acclimation before stocking into the various growout systems (ponds, raceways, tanks) is a critical – and often overlooked, sometimes taken for granted – step in the shrimp culture process. Various water quality parameters should be changed slowly so that the young shrimp have the time to gradually adapt to the new conditions.


