Calcium carbonate, magnesium carbonate essential in management of production ponds

Agricultural limestone, made by crushing limestone to a fine particle size, and lime made by burning limestone in a kiln are widely used in aquaculture. Limestone is comprised of calcium carbonate (CaCO3) and magnesium carbonate (MgCO3) in various proportions. Limestone consisting only of calcium carbonate is called calcite, while that consisting of equal proportions of calcium and magnesium carbonate is known as dolomite.
Pure calcite and dolomite are rare in nature, and most limestone is a mixture of calcium carbonate and magnesium carbonate in which the ratio CaCO3:MgCO3 is greater than 1. Nevertheless, limestone that is predominately calcium carbonate is commonly sold as calcitic limestone, while limestone that has nearly equal proportions of calcium and magnesium carbonates is sold as dolomitic limestone (Table 1).
| Name | Ca (%) | Mg (%) |
|---|---|---|
| Pure calcitic limestone | 40 | 0 |
| Calcitic limestone | 38-40 | <1.2 |
| Pure dolomitic limestone | 21.7 | 13.2 |
| Dolomitic limestone | <20.3 | >12 |
| Ordinary limestone | Other compositions | Other compositions |
Agricultural limestone has the same chemical composition as did the limestone crushed to make it. Burning at high temperature in a kiln drives carbon dioxide from limestone leaving a residue of calcium and magnesium oxides (CaO and MgO) commonly called burnt or unslaked lime. Burned lime can be converted to calcium and magnesium hydroxides [Ca(OH)2 and Mg(OH)2] by treating with water. This product often is called hydrated lime or slaked lime. Of course, when burnt lime is applied to pond water, it immediately reacts with water to become calcium hydroxide.
Liming products used in aquaculture
Several other products are sometimes used in aquaculture for liming. The most common are calcium silicate (CaSiO3) and sodium bicarbonate (NaHCO3).
Liming materials are used in aquaculture primarily to neutralize acidity in bottom soil and water and to increase the total alkalinity of water. Such conditions typically occur in ponds in humid regions with highly-leached, acidic soils. Alkalinity in most waters is primarily from bicarbonate (HCO3–), but at pH above 8.3, there will be some carbonate (CO32-).
Soil acidity usually results from aluminum ions. Aluminum ions, along with other positively-charged ions, are attracted to negative charges on clay particles and soil organic matter. Aluminum ions enter water around soil particles and hydrolyze yielding hydrogen ion (H+) and insoluble aluminum hydroxide ([Al(OH)3] that precipitates. The hydrogen ions make soil pH decline.
All aluminum ions attracted to soil do not enter the water. An equilibrium is reached in which most of the ionic aluminum is on the soil and only a minute amount of it is in the surrounding water. The greater the proportion of aluminum ions to the sum of calcium, magnesium, sodium, and potassium ions attracted to negative charges on soil, the lower the soil pH.
Liming materials react with soil acidity as illustrated below with calcium carbonate:
CaCO3 + 2H+ = Ca2+ + CO2 + H2O
Calcium ions released by the reaction replace aluminum ions on the soil. The displaced aluminum ions hydrolyze in water to produce hydrogen ions, and the reaction with calcium carbonate continues. The upshot is that calcium (and magnesium) ions from liming material replace the aluminum ions on soil and neutralize the acidity caused by hydrolysis of aluminum ions.

Liming material reacts with carbon dioxide in pond water to increase bicarbonate (and alkalinity) as illustrated below for calcium carbonate and calcium silicate:
CaCO3 + CO2 + H2O = Ca2+ + 2HCO3–
CaSiO3 + 2CO2 + 3H2O = Ca2+ + 2HCO3– + H4SiO4.
The alkalinity concentration that can be achieved by these reactions depends upon the availability of carbon dioxide. At the carbon dioxide concentration present in freshwater in equilibrium with atmospheric carbon dioxide, the highest alkalinity achievable by liming with agricultural limestone is about 60 mg/L. Of course, pond waters may contain more carbon dioxide than the equilibrium concentration with air because of organic matter decomposition. Thus, higher alkalinities often can be achieved by liming.
Neutralizing pond bottom soil acidity
To affect a prolonged increase in alkalinity, it is necessary to neutralize bottom soil acidity to avoid it from neutralizing the increase in alkalinity caused by liming. Of course, there are other continuing sources of acidity in ponds, and liming must be repeated periodically. Liming rates for ponds usually are between 1,000 and 5,000 kg/ha.
Lime reacts with carbon dioxide in water to form carbonates as illustrated below for calcium hydroxide:
Ca(OH)2 + CO2 = CaCO3 + H2O.
Calcium carbonate then reacts with carbon dioxide to provide bicarbonate (alkalinity). Thus, carbon dioxide availability also limits the alkalinity increase possible through lime application.
The initial reaction of lime in water can cause a very high pH. Thus, it should only be applied to ponds containing shrimp or fish at rates of around 50 kg/ha. Applications of 2,000-3,000 kg/ha of lime to bottoms of empty ponds can increase soil pH to 12-13. This is why lime treatment of pond bottoms between crops often is used to kill unwanted organisms including vectors of disease. Of course, the high soil pH from lime application subsides very quickly as a result of lime reacting with carbon dioxide. The pH resulting from agricultural limestone addition to water will not exceed 9.
Calcium silicate solubility has the same general relationship to carbon dioxide concentration as does the solubility of agricultural limestone. However, the increase in alkalinity possible with calcium silicate is slightly less than that achievable with agricultural limestone.
Sodium bicarbonate does not behave like other liming materials. It is highly soluble and increases bicarbonate concentration (alkalinity) immediately. This quick reaction is the reason that sodium bicarbonate is the preferred treatment for restoring alkalinity neutralized by acidity from nitrification in highly-intensive culture systems.
Liming materials – other than sodium bicarbonate – will not dissolve appreciably in most freshwaters with more than 60 mg/L alkalinity. In seawater, the alkalinity is nearly 120 mg/L, and seawater usually is near saturation with calcium carbonate. The liming material frequently applied to ponds filled with brackishwater, especially with seawater, usually does not dissolve.
Quality of liming materials
The quality of liming material is assessed mainly by two indicators – neutralizing value and particle size. Lime is usually a fine material and particle size is not often an issue. The neutralizing value is a measure of the amount of acid a given weight of liming material will neutralize. Calcium carbonate is used as the reference and assigned a neutralizing value of 100 percent.
Neutralizing values of some pure compounds representing liming materials are provided (Table 2). To illustrate the use of the neutralizing value, dolomite (calcium magnesium carbonate) will neutralize 108 percent more acidity than will an equal weight of calcium carbonate. Calcium hydroxide is about 135 percent more effective than pure calcium carbonate in neutralizing acidity. In practice, neutralizing value is determined in a chemical test. An agricultural limestone with a neutralizing value of 75 percent would be inferior to another agricultural limestone with a neutralizing value of 92 percent.
| Compound | Neutralizing value (%) |
|---|---|
| Calcium carbonate | 100 |
| Calcium magnesium carbonate | 108.5 |
| Calcium oxide | 178.5 |
| Calcium hydroxide | 135 |
| Calcium silicate | 86 |
| Sodium bicarbonate | 59.5 |
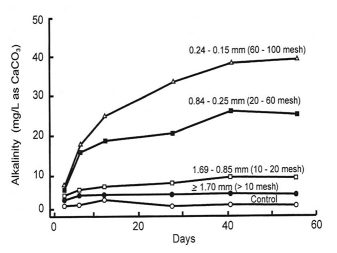
Smaller particles of agricultural limestone are more effective than larger ones because they react faster in water. The concentrations of alkalinity in water resulting from different particle sizes of agricultural limestone over a 2-month period are shown in Table 3. Finer limestone dissolves much faster than coarser limestone.
| Particle size (mm) | Alkalinity (mg/L) |
|---|---|
| 2 – 0.85 | 5.7 |
| 0.84 – 0.42 | 12.2 |
| 0.41 – 0.025 | 26.6 |
| 0.024 – 0.150 | 45.6 |
| 0.149 – 0.106 | 49.6 |
| 0.105 – 0.075 | 55.2 |
Author
-

Claude E. Boyd, Ph.D.
School of Fisheries
Aquaculture and Aquatic Sciences
Auburn University, AL 36830 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Health & Welfare
The importance of iron in aquaculture systems
Iron is the fourth most abundant element in the earth’s crust, but occurs at very low concentrations in surface waters and oceans. It is an essential element for many organisms as part of many enzymes, and also has an important role in plant photosynthesis.

Responsibility
Pond bottom dry-out and liming, part 1
The main pond bottom soil management practices used in semi-intensive culture are pond dry-out and liming between crops. These practices accelerate organic matter decomposition, neutralize soil acidity and destroy unwanted organisms.

Responsibility
Pond bottom dry-out and liming, part 2
The authors believe that dry-out periods likely destroy most organisms in pond bottoms, and liming of the entire bottom area should be done only to neutralize soil acidity and increase pH for organic matter decomposition by soil microorganisms.

