New European food safety regulation based on threshold of toxicity

Setting scientific and policy standards that benchmark the benefits and risks of foods is of great consequence for industry, policymakers and consumers. As the authors have reported previously, precaution plays a pronounced role in the European approach, of which Regulation 178/2002/EC is the core regulatory framework in European food law.
Precautionary themes, regulations
Scientific uncertainty, provisional risk management and policy review seem the precautionary characteristics to assure the “high level of health protection chosen in the Community.” One such precautionary topic is to forestall exposure to antibiotics used in animal rearing and aquaculture beyond a certain limit.
The chloramphenicol episode in the first half of the 2000s launched a debate about the limits of science and technology, and the risks and uncertainties of low-level antibiotics exposure through food consumption, especially when genotoxic carcinogenicity is involved, as is the case with the SEM example.
The detection in 2001 of chloramphenicol in shrimp imported into the Netherlands from Asian countries was regarded as a food scandal. The initial European response was to close European borders to fish products from these countries and make laboratories work overtime to analyze numerous batches of imported goods for the presence of this antibiotic. Some European countries went so far as to have food products containing the antibiotic destroyed.
The actual measurement of low-level concentrations in food, irrespective of toxicological and pharmacological concerns at the consumer level, heightened the awareness and anxiety of the presence and exposure to ostensible man-made chemicals in food.
Subsequent E.U. regulation has not fundamentally amended the regulatory situation that arose in the mid-2000s. Although the new regulation points at the “scientific and technical progress” by which the “presence of residues of veterinary medicinal products in foodstuffs” is detected “at ever-lower levels,” a fundamental solution is not given other than making the minimum required performance limit (MRPL) level – whatever low concentration levels regulatory laboratories in the European Community can detect and confirm – an explicit level of concern.
Although the E.U. officially moved away from zero tolerance, laboratory competence is still the primary deciding regulatory factor in terms of accepting or rejecting food products. Again, “scientific and technical progress” instead of toxicological relevance is the determining factor in regulating chemical compounds deemed undesirable in foods. Put differently, the E.U. does not decide upon health issues with respect to the presence of certain chemical compounds but on the detectability thereof.
Threshold of concern
In July, the European Food Safety Authority (EFSA) proposed the use of a “threshold of toxicological concern” for chemicals with a known structure and for which the exposure is anticipated to be very low. This approach is founded on the principle that the likelihood of toxicity is related to the extent and duration of exposure to a substance. For many types of toxic effects, a dose threshold below which adverse effects are not observed can be identified in experimental studies.
Based on extensive published data on the toxicity of chemicals that have been tested, generic human thresholds of exposure for chemicals (“TTC values”) have been established for groups of substances of similar chemical structure and likelihood of toxicity. Taking a cautious approach, chemical structures have been grouped into three broad categories defined as of low, moderate or high toxicity.
Untested substances can be conservatively assessed by comparing the appropriate TTC value with reliable human exposure data. If human exposure to a substance is below the relevant threshold of concern for its structural class, the likelihood of adverse effects is considered very low.
The EFSA wants to discuss the possibility of making wider use of this risk analysis tool to provide a food safety standard for certain chemicals aimed at consumer health when there are no available scientific data, such as information on low-level exposure. This should, by extension, provide a strong regulatory tool to rule out food safety issues on naturally occurring chemicals with toxic effects at high concentrations, such as chloramphenicol, but also semicarbazide.
Perspectives
This recent EFSA proposal moves away from the precautionary approach that has dominated so many episodes of food safety scares over the past decade and a half. But a lot of work remains to be done.
Many food-endogenous chemicals that have so far not been identified toxicologically would not be covered by the TTC approach, whereby the danger of a new food scare is not actually averted. Many food-endogenous components might be characterized as cancer-inducing at high doses, whereas the usual low-dose exposures through food do not seem to indicate any problems. Chloramphenicol is such an example, which was shown in 2010 to be naturally occurring.
The authors predicted this in 2003. It is therefore essential that TTC values be further developed for compounds such as chloramphenicol that are suspected carcinogens with a predominantly natural origin, of which there are many in our foods at very low concentrations.
Nevertheless, combined with further scientific efforts to define TTC more accurately, we seem to be going in the right direction. Consumer safety and a level global economic playing field on which market entry and continuance can be guaranteed for not only the wealthy countries seem to be merging to the benefit of all.
Editor’s Note: Dr. Jaap C. Hanekamp is also an adjunct professor at the University of Massachusetts Amherst, Environmental Health Sciences; and chairman of the Chemical Food Safety and Toxicity Working Group of the Global Harmonization Initiative.
(Editor’s Note: This article was originally published in the September/October 2012 print edition of the Global Aquaculture Advocate.)
Authors
-
Dr. Jaap C. Hanekamp
Assistant Professor
Roosevelt Academy, Middelburg
Lange Noordstraat 1 4330 A.B.
Middelburg, The Netherlands[108,110,46,99,97,111,114,64,112,109,97,107,101,110,97,104,46,106]
-
Dr. Jan Kwakman
Seafood Importers and Processors Alliance
Kontich, Belgium
Tagged With
Related Posts

Health & Welfare
Ammonia toxicity degrades animal health, growth
Ammonia nitrogen occurs in aquaculture systems as a waste product of protein metabolism by aquatic animals and degradation of organic matter, or in nitrogen fertilizers. Exposure can reduce growth and increase susceptibility to diseases in aquatic species.

Health & Welfare
Nitrite toxicity affected by species susceptibility, environmental conditions
Nitrite, an intermediate compound in the oxidation of ammonia nitrogen to nitrate by nitrifying bacteria in soil and water, is considerably more toxic than nitrate. Exposure to nitrite causes gill lesions and edema in the skeletal muscles of fish, and also affects respiration.
Innovation & Investment
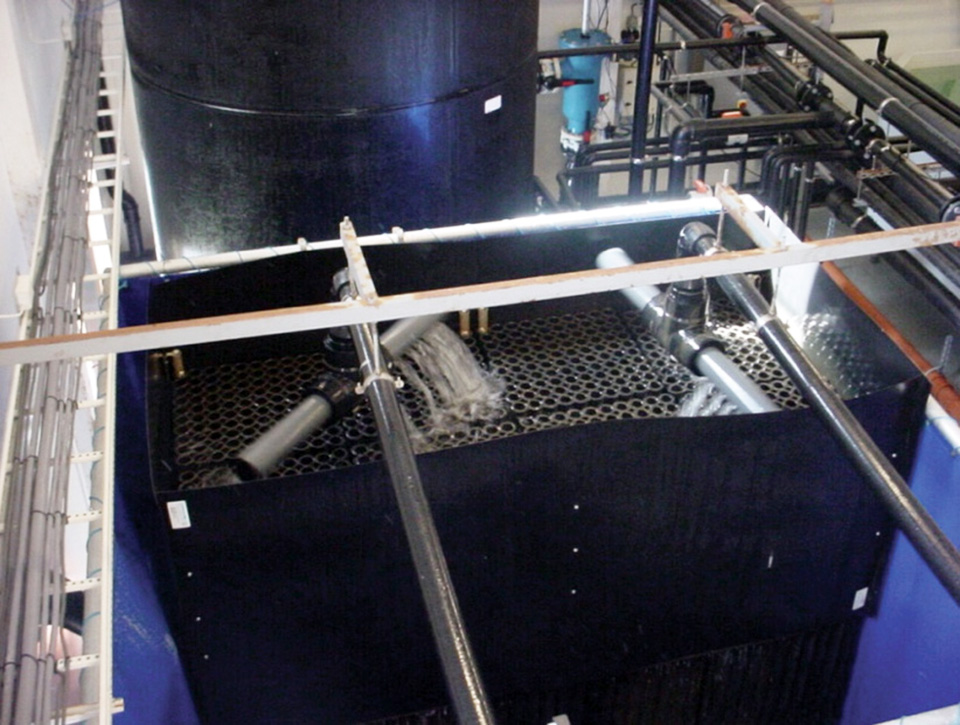
A review of unit processes in RAS systems
Since un-ionized ammonia-nitrogen and nitrite-nitrogen are toxic to most finfish, controlling their concentrations in culture tanks is a primary objective in the design of recirculating aquaculture systems.
Innovation & Investment
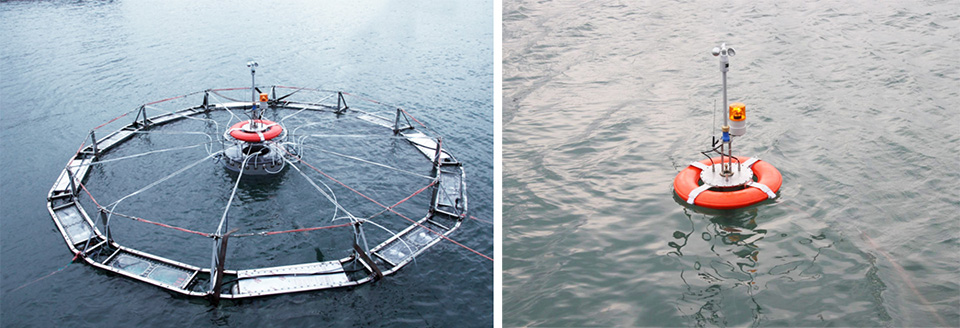
Automatic submersible fish cage systems counter weather, surface problems
The development of submersible fish cage technologies may be necessary to avoid the operational challenges of surface-based aquaculture, which can include extreme temperature and weather conditions, jellyfish infestation, oil spills and many types of biofouling.


