Study reveals risks in WSSV-infected shrimp
Following the introduction and severe impact of white spot syndrome virus in the Americas, the shrimp industry, national governments, and international agencies are concerned that other potentially serious pathogens might follow. The route or routes that led to the introduction of WSSV in the southeastern United States, and later in Central and South America, are still uncertain and controversial.
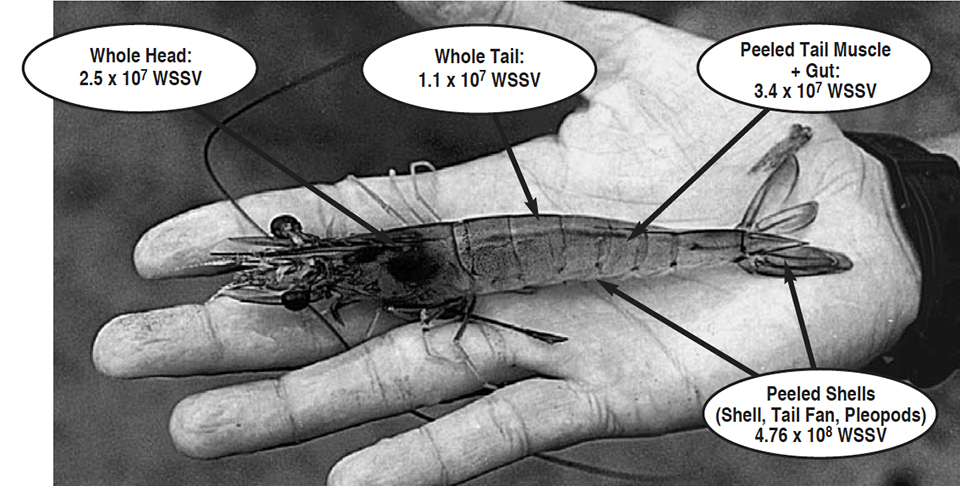
In recent years, U.S. research at the University of Arizona in Tucson has helped identify some of those routes. The university’s current study – supported by the U.S. Marine Shrimp Farming Consortium, U.S. Department of Agriculture, and the National Fisheries Institute – indicates the harvested shell-on tails of WSSV-infected shrimp contain about the same viral load as the heads, and can therefore provide a significant route of disease transmission.
Research rationale
Small-count-size frozen tails of Penaeus monodon with gross signs of acute-phase WSSV infections (likely from emergency harvests) have been common in retail outlets in the United States since as early as 1994. In laboratory studies, such products have been found to transmit WSSV and yellow head virus (YHV) to specific pathogen-free (SPF) indicator shrimp in laboratory studies.
Recent risk assessments in the United States and Australia addressed the risk of introduction and establishment of exotic shrimp pathogens (such as WSSV, YHV, and others) that are posed by various routes. Among the routes and vehicles that were considered in the U.S. risk assessments were shrimp aquaculture (i.e., live shrimp), shrimp processing and other vehicles such as ballast water from vessels and even human sewage.
The conclusions reached in the U.S. assessment were severely limited by the paucity of available data and controversy over the data that were available. Several topics were identified as needing additional research effort:
- improved diagnostic methods
- experiments to reduce uncertainties surrounding virus transmission and virulence
- viral persistence
- monitoring of imported shrimp.
Diagnostic and detection methods
For the three diseases of penaeid shrimp identified as “notifiable” by the Office Internationale des Épizooties (World Animal Health Organization) – WSSV, YHV and TSV – and several of the OIE “other significant diseases,” reliable, sensitive, internationally recognized diagnostic and detection methods have been developed. Laboratory methods are now available to determine the virus content or load of shrimp tissues.
The in situ hybridization method for WSSV has been widely used as a diagnostic method and research tool to determine tissue distribution of WSSV in infected hosts. Quantitative polymerase chain reaction (PCR) methods include competitive PCR and a newly developed technique called realtime PCR. Because of its accuracy and relative ease of use, real-time PCR was used in the present study to determine the WSSV content of whole shrimp, shrimp heads and shrimp tails.
Experimental infection
In this University of Arizona study, small juvenile Penaeus vannamei were experimentally exposed to WSSV in the laboratory and subjected to a simulated emergency harvest when mortalities due to the disease were first detected. This was accomplished by stocking 18 and 16 SPF Penaeus vannamei (average weight 3 grams) in two, 40-liter laboratory aquariums (Tank A and Tank B, respectively) filled with artificial seawater.
Experimental shrimp were fed minced WSSV-infected tissue (1994 isolate from Thailand) at 5 percent (Tank A) and 2.5 percent (Tank B) of body weight for two days. Following this feeding, the shrimp were maintained on a commercial pelleted ration. They began to die three days after exposure.
After the first WSSV mortalities were observed, the remaining shrimp were collected to simulate an emergency harvest. Representative shrimp taken on day 0 of the study served as negative control samples, while the harvested shrimp taken at day 3 were either fixed in Davidson’s AFA fixative for histological and in situ hybridization analysis or frozen at minus-70 degrees-C for real-time PCR analysis.
Histology and in situ hybridization
The results from histological sections processed using routine H&E staining and in situ hybridization with a WSSV probe showed three of the four WSSV-exposed shrimp sampled presented advanced systemic WSSV infections. Using the grading scheme for infection severity from Lightner (1996), the infection severity of the five experimental shrimp was grade 0 for one shrimp and grade 3 to 4 for the remaining three specimens.
In the three shrimp with advanced WSSV infections, characteristic WSSV basophilic intranuclear inclusion bodies were abundant in the cuticular epithelium and connective tissues of the general body cuticle and appendages (i.e., the carapace, abdominal tergal and pleral plates, gills, antenna, maxillipeds, pereiopods, pleopods, telson, and uropods). The bodies were also found in the foregut (esophagus and anterior and posterior chambers of the stomach), and in the hindgut and rectum (Fig. 2).
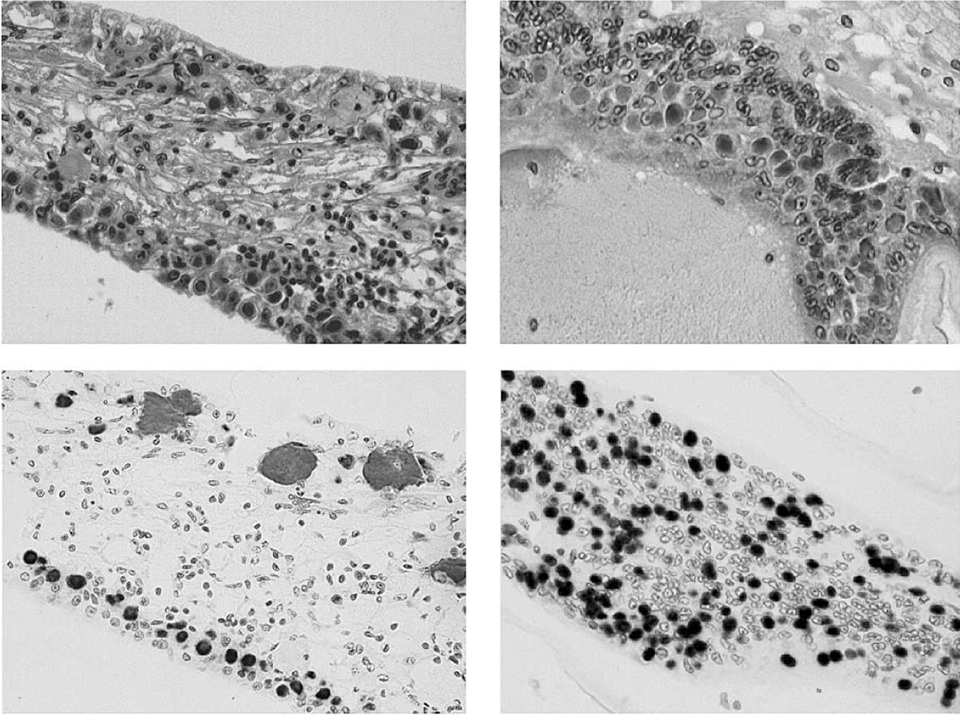
With direct comparisons of the head and tail sections from the same shrimp, no difference in severity of WSSV infection was apparent in the cuticular epithelium present in the head (i.e., stomach and head appendages) or tails (i.e. pleopods, uropods, and telson). WSSV inclusions were also apparent – but generally in lower numbers – in the circulating hemocytes, fixed phagocytes, antennal gland tubule epithelial cells, lymphoid organ sheath cells, parenchymal tissues of the hematopoietic tissues, and connective tissues throughout the shrimps’ bodies.
Real-time PCR
For each run, a standard curve was generated from samples of purified cloned WSSV plasmid ranging from 4.00×107 to 400 copies (Fig. 3). Copy numbers were calculated by interpolation of the experimentally determined threshold cycle (CT) as previously described for IHHNV by Tang and Lightner (in press). From these copy numbers, the final results were expressed as the mean copy number of WSSV per microgram of total DNA.
The same proportion of low-grade to advanced WSSV infections found in histological assays would be expected in the 16 post-harvest shrimp assayed for WSSV by real-time PCR. This inherent variability in viral load was apparent in the PCR findings. However, since the data were paired (using the viral content of heads and tails from the same shrimp), the effect of stage of infection on viral content was minimized.
Whole heads and tails
The mean WSSV copy number in whole heads was found to be slightly greater than the mean in whole tails. From one-fourth to two times more WSSV copies were found in the head than in the tail when the WSSV copy numbers were expressed per microgram of total DNA. These values were not statistically different at a 95 percent confidence level, but were different at a 90 percent level of confidence. No difference was found between WSSV copy number between Tank A and Tank B.
The relative ratio of the total body weight of the head versus tail in most penaeids is about 40:60. In P. vannamei, the shrimp’s tail makes up about 58 percent of its total weight, while the head makes up about 42 percent. When the WSSV copy number values, expressed as number per gram of tissue, are compared and extrapolated to this 42:58 body weight ratio, we find that 51 percent of the WSSV load is in the tail and 49 percent is in the head.
The viral load of the head and the tail are similar, because both the head and tail of shrimp include large amounts of the principal target tissues (cuticular epidermis, connective tissues, hemocytes, and hemolymph) that are heavily infected in acute WSSV infections.
Peeled shells
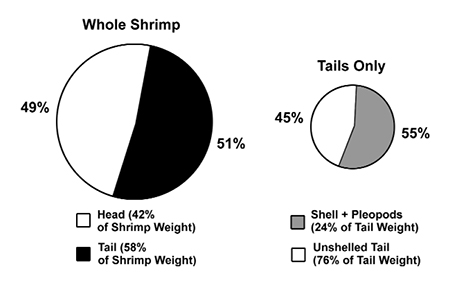
The WSSV copy number mean was between four and 10 times greater in the abdominal shells than in the tail muscle (significant at 95 percent level of confidence). On a perweight basis, peeled shells from the tails (with pleopods and tail fan) contain almost 10 times more WSSV than do whole heads or whole tails (Fig. 4). In proportion to the total tail weight, the virus load of the peeled shell represents 55 percent of the total viral load in tail. Therefore, when the shrimp are unshelled, 45 percent of the total virus load of the tail remains in the edible tail meat, while 55 percent of the virus content of the tail is present in the peeled shell, which may become part of the processing waste.
Conclusion
The present study confirms that small-size “green frozen shrimp tails” from emergency harvesting due to the onset of WSSV contain large amounts of the virus. The results also support the conclusions of the Australian risk assessment, which concluded that shrimp from emergency harvests pose a greater risk of pathogen introduction than healthy shrimp. However, the results do not support the assumption of U.S. and Australian risk assessments – that shrimp tails pose a lower risk than whole shrimp or shrimp heads.
Shell-on tails of emergency-harvested shrimp that are infected by WSSV contain about the same viral load as the heads. When tails are peeled, about 55 percent of the viral load is removed. Thus, a total of 77 percent of the viral load of heavily infected whole shrimp is contained in the whole head and peeled shell, which may become part of the processing waste. This emphasizes the importance of proper disposal of processing waste to avoid viral disease transmission.
(Editor’s Note: This article was originally published in the December 2000 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-

Don Lightner, Ph.D.
Department of Veterinary Science and Microbiology
University of Arizona, Tucson, USA[117,100,101,46,97,110,111,122,105,114,97,46,117,64,108,118,100]
-
S.V. Durand
Department of Veterinary Science and Microbiology
University of Arizona, Tucson, USA -
R.M. Redman
Department of Veterinary Science and Microbiology
University of Arizona, Tucson, USA -
L.L. Mohney
Department of Veterinary Science and Microbiology
University of Arizona, Tucson, USA -
K. Tang-Nelson
Department of Veterinary Science and Microbiology
University of Arizona, Tucson, USA
Tagged With
Related Posts

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
Biosecurity principles for sustainable production using SPF shrimp
Basic components of biosecurity include knowledge of diseases, adequate detection methods and the use of “clean” shrimp stocks.

Health & Welfare
The microsporidium <i>Perezia</i> sp. and cotton shrimp disease
Study analyzes samples of microsporidia-infected shrimp in Madagascar, Mozambique and Saudi Arabia and with clinical signs of cotton shrimp disease.


