Product not useful for ammonia removal
Zeolite is a naturally occurring mineral that also can be produced synthetically. It is used in industry for water softening and as media for molecular sieves. In recent years, there has been interest in possible agricultural and aquaculture applications.
In aquaculture, zeolite can be used in filters for removing ammonia from water in tanks that hold and haul fish. Zeolite is also commonly applied to shrimp culture ponds in southeast Asia and some other regions. The material is broadcast over pond surfaces at weekly to monthly intervals. In spite of its popularity as a pond treatment, however, there has been little research on the effectiveness of zeolite in shrimp culture.
Zeolites in nature
Zeolites are mined from natural deposits in many nations. There are more than 50 types of natural zeolite and a variety of artificial zeolite compounds.
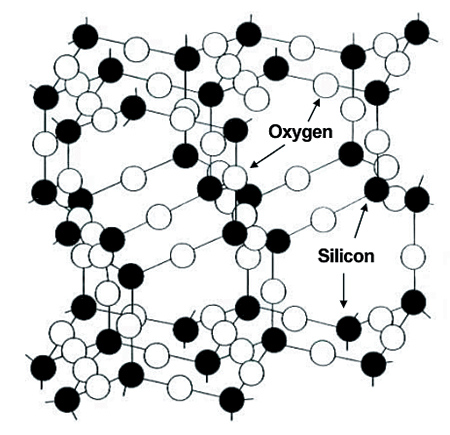
Natural zeolites are aluminosilicate clay minerals with rigid three-dimensional frameworks of silicon and aluminum oxide tetrahedrons honeycombed by corridors large enough for water or gas to enter (Fig. 1). Their unique tetrahedron structure leaves unsatisfied negative ionic charges within and on the surfaces of zeolite crystals.
In nature, these charges are satisfied mostly by sodium. Because the sodium ions can be exchanged for other cations such as potassium, calcium, magnesium, and ammonium in solution, zeolites are used widely as cation-exchange media in applications like water softening. In water softening, sodium ions of zeolite exchange for calcium and magnesium ions in water.
Selective adsorption
The large internal cavities in zeolite easily fill with water, air, or other molecules. They have strong capacities to adsorb and desorb molecules that are small enough to pass through the entry channels. Their adsorptive surface area is up to 300 m2 per grams zeolite, and some zeolites can adsorb up to 30 percent of their weight in gases and other molecules. Because of their ability to selectively adsorb molecules of specific sizes, zeolites are used in molecular sieves.
Artificial zeolites have properties similar to natural ones, but no advantages over natural zeolites for use in aquaculture.
Benefits incorrectly alleged
Shrimp farmers expect zeolite to remove ammonia from pond water. Some also think it will remove hydrogen sulfide and carbon dioxide, and serve as a source of silica for diatoms.
Ammonia removal
In shrimp pond water, ammonium concentrations usually range 1 to 5 milligrams per liter. Concentrations of sodium, potassium, calcium, and magnesium ions (Table 1) are much greater than the ammonium concentration. These cations compete with ammonium for exchange sites on zeolite, and the ability of zeolite to remove ammonium from water is much lower than the total capacity of the mineral to absorb cations.
Boyd, Table 1
| Salinity | Ammonia Removal (g/kg zeolite) |
|---|---|
| 0.1 ppt (tap water) | 9.0 |
| 4 ppt | 0.12 |
| 8 ppt | 0.10 |
| 16 ppt | 0.08 |
| 32 ppt | 0.04 |
For example, the author once conducted a simple laboratory trial to determine the influence of salinity on ammonia removal by zeolite from water. The results follow.
The ability of zeolite to remove ammonia declined rapidly with increasing salinity. At a salinity of 4 ppt, the removal of ammonia by zeolite was only 1.3 percent of the removal achieved in tap water.
Intensive shrimp ponds in Asia often are about 1.5 meters in depth and 1 ha in area, and contain about 15,000 cubic meters of water. Suppose a pond manager applied 50 kilograms per hectare zeolite to decrease the ammonia concentration in a pond with 4 ppt salinity. The amount of ammonia nitrogen removed by the zeolite can be estimated as follows:
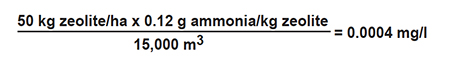
The amount of ammonia removed would be insignificant. In fact, removal of 1 milligrams per liter ammonia from the hypothetical pond would require a zeolite application of 125,000 kg. Even in freshwater, about 1,667 kg of zeolite would be necessary to remove 1 milligrams per liter ammonia from a 15,000-cubic-meter pond.
Zeolite is therefore not a useful treatment to remove ammonia from ponds. The costs of product and labor to apply it at the high rates needed for effective action are prohibitive.
Of course, zeolite can be used to remove ammonia from smaller-scale holding or hauling tanks. The tank water can be passed through a large filter for ammonia removal and sent back to the tank. When the filter is saturated with ammonium, it can be recharged by flushing with a brine solution to exchange ammonium for sodium.
Gas removal
When zeolite is placed in water, its internal cavities fill with water and cannot effectively absorb gases. The capacity of zeolite to remove gas from water is limited to the gas dissolved in the water that enters the voids in the mineral structure.
The void volume or “open space” in zeolite ranges 30 to 50 percent. The bulk density of typical zeolite is around 2 grams per cubic centimeter, so each gram of zeolite would have about 0.15 to 0.25 ml of voids. A kilogram of zeolite could absorb about 150 to 250 ml of water. If the water contains 1 milligrams per liter hydrogen sulfide, a kilogram of zeolite could remove 0.15 to 0.25 mg hydrogen sulfide.
A treatment of 50 kg could remove 0.0075 to 0.0125 grams of hydrogen sulfide and reduce the hydrogen sulfite concentration in a 15,000-cubic-meter pond by 0.0000005 to 0.0000008 milligrams per liter. This tiny reduction in hydrogen sulfide concentration would not be noticeable. Similar minimal effects would apply to the removal of other gases.
Silica source
Since the silicon in zeolite is not water-soluble, it is unavailable to diatoms. Besides, pond bottoms usually are constructed of aluminosilicate clay, and adding a bit more aluminosilicate in zeolite would not increase silicon availability.
Conclusion
The properties and reactions of zeolite indicate the substance has little value as a pond treatment. It will not harm water and soil quality or leave undesirable residues in fish and shrimp, but its use is only an unnecessary expense.
(Editor’s Note: This article was originally published in the December 2003 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Professor, Department of Fisheries and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Responsibility
A review of water quality improvement products
Prof. Boyd examines products used by aquafarmers to improve water quality and conditions in their ponds and discusses their efficacy.

Responsibility
Ammonia nitrogen dynamics in aquaculture
The major sources of ammonia in aquaculture ponds are fertilizers and feeds, and problems with high ammonia are most common in feed-based aquaculture.

Health & Welfare
Ammonia toxicity degrades animal health, growth
Ammonia nitrogen occurs in aquaculture systems as a waste product of protein metabolism by aquatic animals and degradation of organic matter, or in nitrogen fertilizers. Exposure can reduce growth and increase susceptibility to diseases in aquatic species.

Responsibility
Does Coriolis force impact aerator placement in aquaculture ponds?
The Coriolis effect has no bearing on aerator placement and aquaculture pond management. The most important consideration with mechanical aeration is to provide a sufficient amount to maintain adequate dissolved oxygen concentration.

