Early detection, appropriate emergency response can arrest progression of an epidemic

Case history
This clinical case report occurred in a Pacific white shrimp (Penaeus vannamei) production facility located on the Pacific Coast of Central America. The acute disease event was in one of five, 100-ton nursery tanks that had been stocked on the same day from a common batch of postlarvae (PL). The stocking density was 1.5 million PL/tank (15 PL/L). Sick and dying PL were not observed (at that time or subsequently) in the four other nursery tanks in the enclosed, nursery building.
The routine, daily management for the five tanks included continuous water flow with 10 to 20 percent water exchange. A commercial, imported 350-micron (particle size) feed crumble was administered every two hours for all stocked tanks in the building. The tank bottoms were not routinely inspected or siphoned to remove accumulated sediment. Probiotic products were not used in the tanks prior to or following the onset of the disease. No other routine chemical or biological management measures were applied in the five tanks.
Clinical observations
The onset of clinical signs occurred at PL16, which was four days after the tanks had been stocked. On the day of the onset of the disease, the baseline water quality parameters included water temperature 28.7 degrees-C, salinity 29 ppt and dissolved oxygen (DO) 5.3 mg/L (permanent aeration with blowers was used). The pH of the water was not measured. The turbidity was considered high but Secchi disc measurements were not recorded, with abundant, suspended clumps of organic matter in the water column of the five tanks. The turbidity was such that the bottoms of the affected, as well as the other nursery tanks, were not visible when the tanks were full.
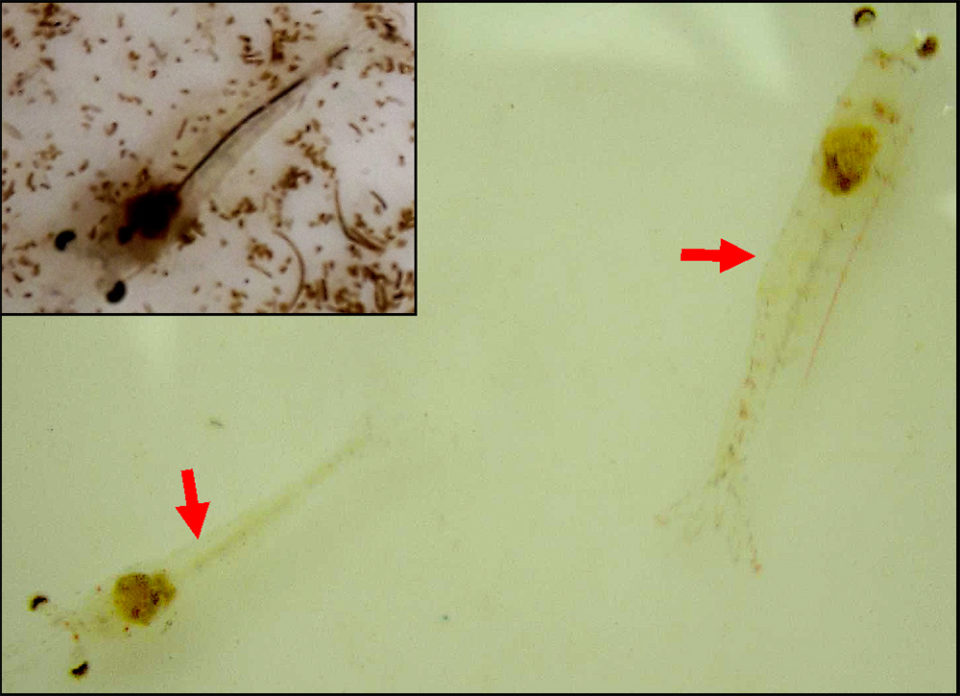
Most of the PL16 appeared unaffected in the tank. The abnormal PL16 had shrunken whitish hepatopancreas (HP), whitish body discoloration, empty intestine, erratic surface swimming and loss of the “escape reflex.” The presumptive diagnosis was AHPND for the disease and the presumptive differential diagnosis was White Spot Disease (WSD) or chemical toxicosis (e.g. ammonia).

At approximately 7 a.m., nursery technicians reported to management that sick and dying PL were observed in tank 6. After consultation and discussion between staff and managers at 10 a.m., the intervention steps in sidebar 1 were initiated:
Intervention steps taken
- The water level was dropped in the tank.
- The accumulated detritus (which was unusually abundant) was siphoned out of tank 6, a 200 percent water exchange was performed and the tank was refilled.
- Thereafter, daily, 100 percent water replacement and bottom siphoning to remove detritus was carried out and if the previously administered feed had been consumed new feed (40 percent protein, high quality PL ration) was reapplied. This was continued until the PL tank was harvested 5 days after the onset.
As the tank intervention progressed, staff collected and preserved samples of moribund PL for PCR and histology analyses.
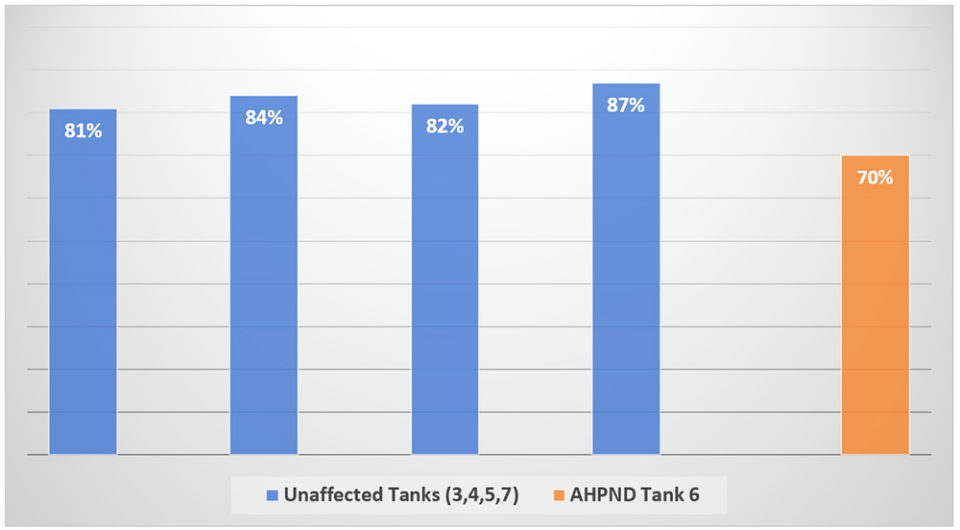
Within hours of remediation, PL mortality had decline to below detection by visual observation. Staff did not observe further PL mortality the next day or thereafter. Five days after the onset of the disease the five tanks were harvested. The PLs in all tanks appeared robust and active. The harvest survival was 70 percent for the AHPND-affected tank and the mean ± sd survival was 83.5 ± 0.027 percent for the four unaffected nursery tanks (Fig. 1).
Laboratory analysis findings, discussion
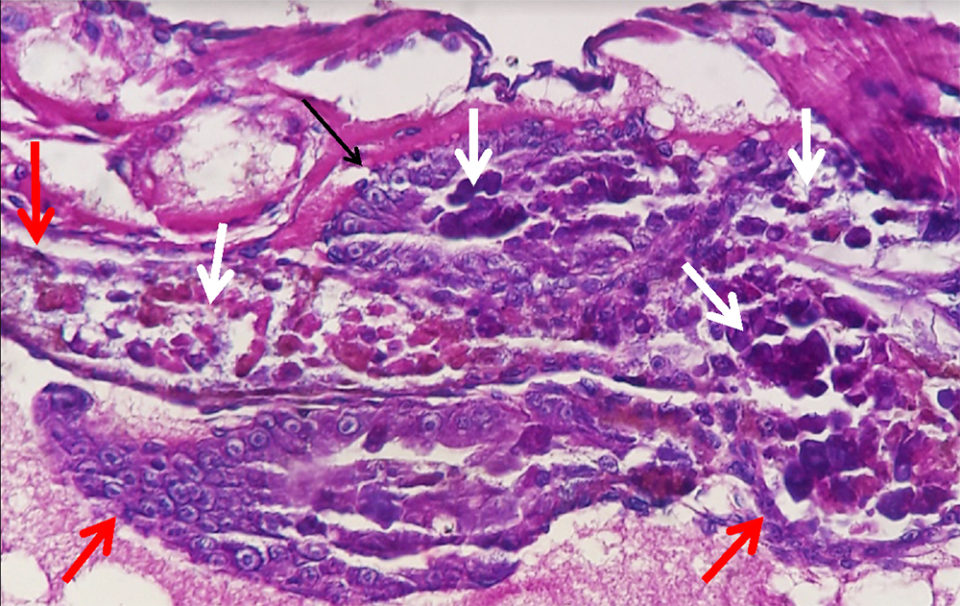
The laboratory report stated that both the AP4 PCR and histopathology tests were positive for AHPND. There were no other significant laboratory findings.
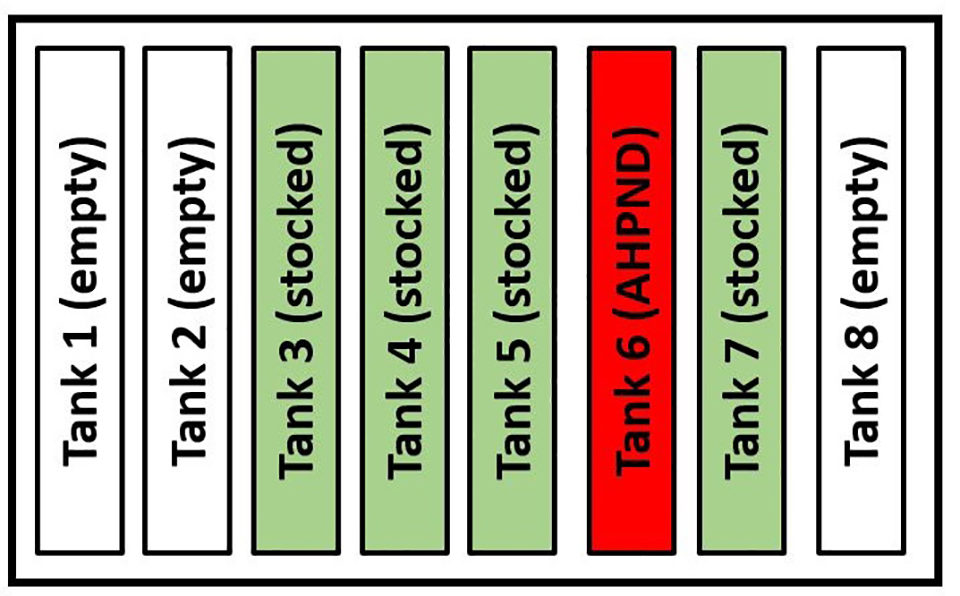
Diagram 1 shows the physical arrangement of the eight tanks in the nursery building and the location of tank 6 in relation to the other tanks. Tank 6 was the only tank impacted by AHPND. Because only one tank was compromised by AHPND and not the other four tanks, which had been stocked and maintained using the facility’s Standard Operating Procedures (SOP), the attack pattern revealed that the predisposing factor(s) that contributed to AHPND outbreak in tank 6 were isolated to one tank.
Furthermore, it is unlikely that factor(s) common to all tanks (incoming water quality, PL quality when the tanks were stocked, the quality of the feeds applied to all the PL tanks, etc.) nurtured onset of AHPND, but rather the initiating factor was confined only to tank 6. In our opinion, the precipitating factor was accidental overfeeding the night prior to the onset of AHPND in tank 6. As one of us (JAB) and coauthors reported in 2015, uneaten feed was colonized by AHPNDVp and within about 4 hours of incubation the contaminated feed posed a significant (AHPND) risk hazard to shrimp that ingested the colonized and PirA/B toxin contaminated feed.
Sidebar 2 lists substrates that are known to support rapid growth and excretion of the highly noxious Pir A/B toxins by AHPNDVp.
Examples of substrates that promote AHPNDVp excretion of Pir A/B toxins
- Nutrient dense microbiological broth media.
- Organic fertilizers, molasses, excess shrimp feed, etc. applied prior to or after stocking shrimp.
- Uneaten shrimp feed and/or nutrient rich sediments in ponds and tanks.
- Shrimp cadavers.
Additionally, the mortality curve data support the interpretation that Pir A/B toxins were elaborated and accumulated in uneaten feed and organic bottom sediments of the tank. We hypothesize that during the night the PL ingested uneaten, spoiled feed and/or bottom detritus and by doing so, consumed abundant bacteria + harmful levels of Pir A/B toxins.
The Pir toxin genes appear to be upregulated when Pir A/B toxigenic bacteria encounter environmental and nutrient conditions that will support rapid multiplication of the bacteria and provide suitable conditions for quorum sensing and Pir A/B toxin production. We list examples in Sidebar 3 which we believe to be suitable substrates that promote the formation and excretion of these toxins.
Rapid response steps to an AHPND outbreak in a lined-bottom shrimp nursery tank
- This is an animal health emergency! Once recognized, immediate response is essential.
- If necessary, lower the water level in the tank until the bottom of the tank is clearly visible. Continue water replacement if the incoming water lacks significant turbidity.
- Start siphoning to remove accumulated detritus and shrimp carcasses.
- Cease feed application and reduce aeration intensity to allow suspended material to settle. As quickly as practical, siphon the tank bottom to remove accumulated detritus and all shrimp carcasses.
- Collect symptomatic shrimp specimens in appropriate tissue fixative for laboratory tests to confirm or not that the cause is AHPND. Collect shrimp specimens from the siphoned detritus. Shrimp cadavers can be used as samples for AHPND PCR testing.
- Inspect the tank hourly and assess the status. Resume feed application hourly but apply it carefully and reduce the feed amount if uneaten feed remains in the tank.
- Siphon the tank bottom twice daily, or more frequently if needed.
- After three to five days, resume standard SOPs for nursery tank management.
- In regions where AHPND is indigenous, always be aware of the danger of accumulated bottom detritus in shrimp nursery tanks and, if not already a part of tank sanitation SOPs, consider an upgrade to daily or more frequent tank bottom inspection and siphon removal of accumulated detritus/sediment.
Ingestion of Pir A/B toxins caused necrosis and slough of exposed hepatopancreas tubule epithelium. The cellular debris provided a nutrient rich environment which combined with high density of V. parahaemolyticus and, presumably, other Vibrio spp., cascading into a life-threatening disease for the Pir A/B poisoned shrimp. Recently, a severe AHPND disease event with an onset in a hatchery in Latin America was confirmed to be caused by V. owensii using molecular tools (Cuéllar-Anjel, unpublished data).
Historically, prevention of AHPND was recognized by progressive farmers in Thailand and, perhaps, elsewhere as far back as 2013 to 2014. Progressive farmers approached the AHPND problem logically and through trial and error discovered that losses from AHPND could be reduced significantly or largely eliminated through adjustments to feeding (small amount, fed every few hours) coupled with daily or several times daily thorough removal of detritus from the bottom of raceway/tank or lined ponds.
While the case reported herein demonstrates that early detection and implementation of the appropriate emergency response can be effective to arrest progression of an AHPND epidemic, the authors stress that prevention is preferred over emergency intervention in disease management.
Perspectives
This case report highlights that control of AHPND can be achieved in nursery tanks by rapid remedial action (Sidebar 3), when the onset of the disease is recognized early.
For the prevention of AHPND, the authors stress the importance of avoidance of overfeeding and regular inspection and removal of uneaten feed and other organic suspended and bottom sediments from nursery tanks or lined ponds. Nursery tank bottom sanitation is a key to avoidance of AHPND in regions and zones where AHPNDVp is indigenous.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Jorge Cuéllar-Anjel, D.V.M., M.Sc., Cert. Aq. Prof.
Aquaculture Veterinarian
Calle 101 # 20-33 Apt. 301
Bogotá, D.C., Colombia[109,111,99,46,108,105,97,109,103,64,110,97,117,99,111,106]
-
James A. Brock, D.V.M.
Aquatic Farms
49-239 Kamehameha Hwy.
Kaneohe, Hawaii 96744, USA[109,111,99,46,100,117,111,108,99,105,64,51,53,54,50,109,105,106]
Tagged With
Related Posts

Health & Welfare
Four AHPND strains identified on Latin American shrimp farms
Two virulence genes are known to encode a binary photorhabdus insect-related toxin that causes acute hepatopancreatic necrosis disease in shrimp. The pathogenicities of these V. campbellii strains were evaluated through laboratory infection and subsequent histological examination in P. vannamei shrimp.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
Genetic variation for resistance to WSS, AHPND in Pacific white shrimp
Selection for disease resistance has been used in breeding farm animals and can be a viable option to deal with white spot syndrome and acute hepatopancreatic necrosis disease in commercial shrimp culture. In trials, heritability for AHPND resistance was low, while that for WSS was moderate.

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).



